Overview
Abstract
Substances, including drugs, gases, and hormones, are transported through the body within the bloodstream and airflow in the Cardiovascular and Respiratory Systems, respectively. This provides important nutrients and waste removal for normal physiologic function. The engine models advection in both fluid systems (see Substance Transport Methodology). Transvascular transport is simulated using one of several diffusion models (see Tissue Methodology) for substances generically. Drug transvascular transport is modeled with a physiologically-based pharmacokinetic (PBPK) model, and the physiologic effects on the body are modeled with a low-fidelity pharmacodynamic (PD) model.
Introduction
One major category of substances transported through the body is drugs. Drugs influence the body by moving it out of homeostasis for a specific purpose, such as sedation for surgical intervention, or to address a patient condition, such as analgesic intervention. After a drug enters the patient’s body (through intravenous administration, absorption through the gastrointestinal system, or inhalation of an agent), the substance ultimately is transported through the bloodstream for uptake by the tissues and organs. The process of clearing a drug from the system is accomplished in a combination of three methods: renal clearance, hepatic clearance, and systemic clearance. Depending on the drug, it may be processed by either the kidney, the liver, or both for removal from the system as a waste product. Additionally, drugs are metabolized by the body and removed from the bloodstream. These methods all reduce the amount of drugs in the system. The drug transport and clearance methodologies in the engine are described below.
System Design
Background and Scope
Pharmacokinetics is the study of the relationship between the dose of a drug and the time-evolution of the concentration of the drug throughout the body. The relationship between the concentration of a drug and the physiological response is described by the pharmacodynamics [299]. The engine has both pharmacokinetic and pharmacodynamic models. Because it is physiologically-based, the pharmacokinetic model has resolution at the same scale as the cardiovascular and respiratory systems. Physiologically-based pharmacokinetic (PBPK) modeling is a technique that mathematically models the distribution, uptake, metabolism, and clearance of a drug with consideration of the relevant physiological processes [182]. The PBPK model provides more resolution than the classic one and two compartment models. The PBPK model also provides a base on which to build a receptor-based pharmacodynamic (PD) model, although the PD model is currently a lower-fidelity phenomenological model based solely on the concentration of a drug in the blood. Drugs impact body-level physiologic behavior such as heart rate, respiration rate, and blood pressure as result of cellular-level interactions caused by a drug that changes the behavior of physiologic entities, such as blood vessels and electrical activity in the heart. The cellular-level processes are not modeled in the engine, and instead the PD model is a direct relationship between concentration and maximum effect for a body-level response.
Data Flow
Preprocess
Administer Substances and Administer IV Substances
All substances added or removed from the engine body are incremented or decremented from the appropriate compartment during the Preprocess step. For example, if substances are administered via a bolus injection or through intravenous administration, the substance mass is increased in the vena cava compartment.
Process
The generic substance methodology developed for the engine is used to solve for the mass, concentration, substance volume, and volume fraction in each compartment. After the masses and concentrations have been updated, the system-level effects of any circulating drugs are calculated using the pharmacodynamic model.
Post Process
The Post Process step moves everything calculated in Process from the next time step calculation to the current time step calculation. Substances have no specific Post Process functionality. Each system post processes their circuit and moves the mass, concentration, substance volume, and volume fraction from next to current.
Features, Capabilities, and Dependencies
Pharmacokinetic models quantify the time evolution of drug distribution from the administration of a dose. Pharmacodynamic models, on the other hand, characterize the events from the arrival of the drug at the site of action to the onset, magnitude and duration of the biological response [299]. The Drug system contains both pharmacokinetic and pharmacodynamic models.
Pharmacokinetics
The pharmacokinetic (PK) methodology provides a means for simulating the time-evolution of the distribution of a drug throughout the body. This is accomplished by using the administration actions discussed in the Actions section below, the transport methodology, the perfusion limited diffusion methodology, and the substance files discussed in the Common Data Model documentation. Once a drug is administered, it either enters the Cardiovascular System (liquid) or the Respiratory System (inhaled). If it is an inhaled drug, modeled in the engine as a gas, the drug will move into the alveoli through advection then diffuse into the Cardiovascular System through alveoli transfer. Drug effects, described below in the drugs-pharmacodynamics section, are then computed based on the concentration of the drug in the plasma. Note that the plasma concentration calculation is currently incorrect. The concentration of a drug is currently computed by dividing the mass of the drug in a compartment by the volume of plasma, as computed from the hematocrit. Implicit in the plasma concentration calculation is the assumption that all of the drug is in the extracellular space. In actuality, some of the drug will cross blood-cell membranes, and the concentration of drug in the plasma and in the blood is a function of many factors, including the lipophilicity. We will address this error in the future.
Partition Coefficient
The drugs circulate around the cardiovascular circuit via the generic transport methodology described in the Circuit Methodology. However, drugs diffuse from the Cardiovascular System into the tissues via perfusion limited diffusion. For each drug, the physicochemical properties are used to calculate the partition coefficient. This partition coefficient describes the affinity for the particular drug to diffuse across the barrier between the cardiovascular and tissue spaces. Each drug has its individual physicochemical properties described in the substance file with a calculated partition coefficient for each tissue compartment. For a very weak base, an acid, or a neutral, Equation 1 is used to calculate the partition coefficient.
![]()
For moderate to strong bases, Equation 2 is used to calculate the partition coefficient.
![]()
Where X and Y are the different relationships for pH, as shown in Table 1, *fIW* is the fraction of intracellular water, *fEW* is the fraction of extracellular water, *fNP* is the fraction of neutral phospholipids in the tissue, *fNL* is the fraction of lipids in the tissue, P is the octanol:water partition coefficient for the drug, *fu* is the fraction of the drug unbound in plasma, *fNL,P* is the fraction of neutral lipids in plasma, *fNP,P* is the fraction of phospholipids in plasma, and *PRT* / *PRB* is the tissue to plasma ratio of the binding protein.
| Ionic State | X | Y |
|---|---|---|
| Acid |
|
|
| Very Weak Base |
|
|
| Neutral | 1 | 1 |
A number of these values are drug parameters found in the substance file definitions. However, many of them are organ/compartment dependent as shown in Tables 2 and 3.
| Tissue Compartment | Extracellular Water (*fEW*) | Intracellular Water (*fIW*) | Neutral Lipd (*fNL*) | Neutral Phospholipid (*fNP*) |
|---|---|---|---|---|
| Adipose (Fat) | 0.135 | 0.017 | 0.853 | 0.0016 |
| Bone | 0.1 | 0.346 | 0.017 | 0.0017 |
| Brain | 0.162 | 0.620 | 0.039 | 0.0015 |
| Gut | 0.282 | 0.475 | 0.038 | 0.0125 |
| Kidney | 0.273 | 0.483 | 0.012 | 0.2420 |
| Liver | 0.161 | 0.573 | 0.014 | 0.2400 |
| Lung | 0.336 | 0.446 | 0.022 | 0.0128 |
| Muscle | 0.118 | 0.630 | 0.010 | 0.0072 |
| Myocardium | 0.320 | 0.456 | 0.014 | 0.0111 |
| Skin | 0.382 | 0.291 | 0.060 | 0.0044 |
| Spleen | 0.207 | 0.579 | 0.0077 | 0.0113 |
| Tissue Compartment | Tissue to Plasma Albumin Ratio | Tissue to Plasma Lipoprotein Ratio |
|---|---|---|
| Adipose (Fat) | 0.049 | 0.068 |
| Bone | 0.100 | 0.050 |
| Brain | 0.048 | 0.041 |
| Gut | 0.158 | 0.0141 |
| Kidney | 0.130 | 0.137 |
| Liver | 0.086 | 0.161 |
| Lung | 0.212 | 0.168 |
| Muscle | 0.064 | 0.059 |
| Myocardium | 0.157 | 0.160 |
| Skin | 0.277 | 0.096 |
| Spleen | 0.097 | 0.207 |
The implementation of the partition coefficients calculation and all values shown in the tables are derived from the literature [295] [294] [182] [169] [361].
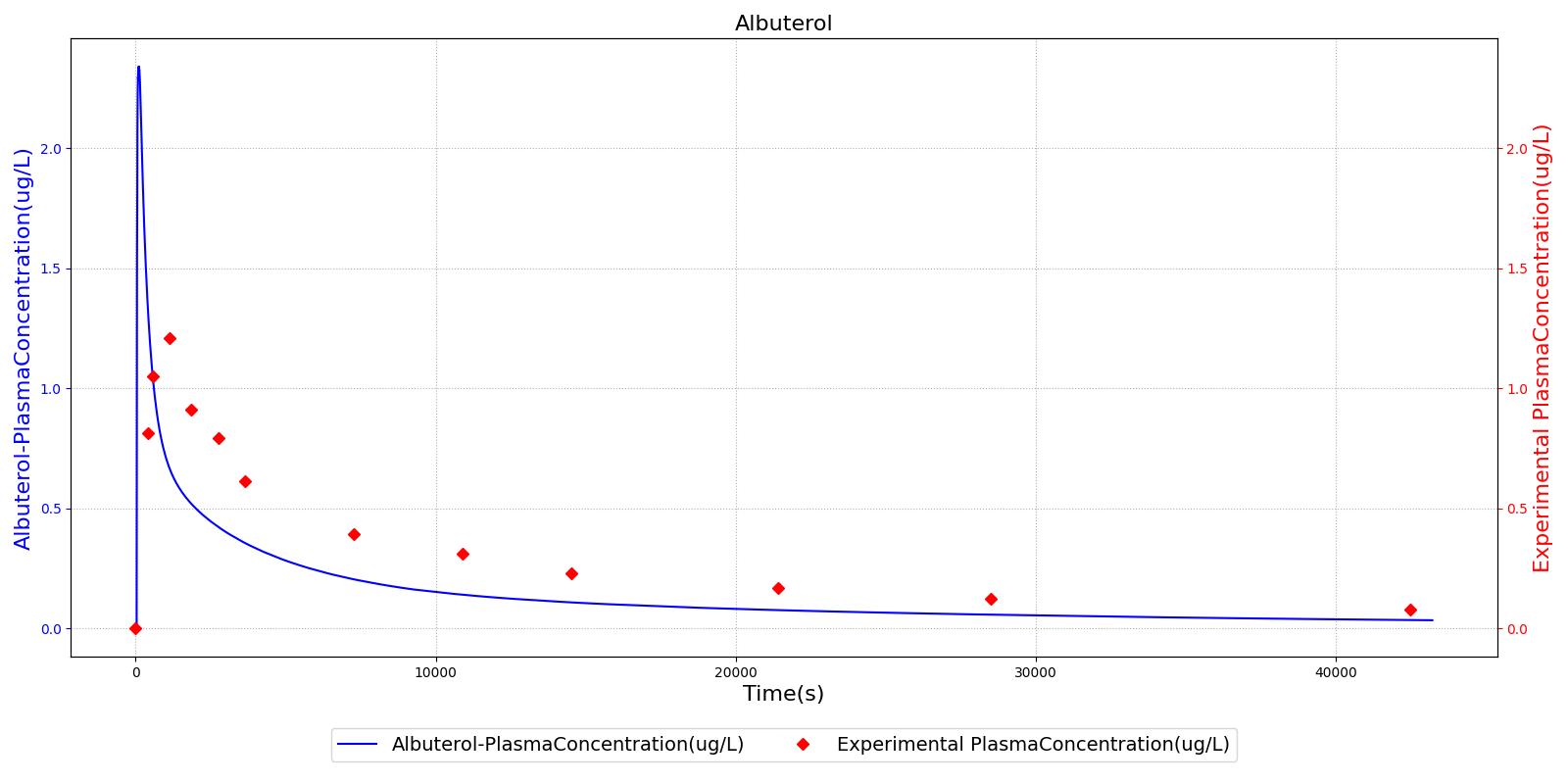
If it is not possible to obtain the information required to compute the partition coefficient for a drug, or if the calculation is not valid for a particular drug for any reason, it is possible to override the computation by directly inputting a constant partition coefficient in the drug file. Note that, to use this functionality, it is best to input a coefficient for every tissue compartment in the model. The engine currently uses the coefficient-computation override methodology for Albuterol, Ketamine, and Naloxone. The parameters used to calculate each partition coefficient were found in published data and/or tuned to get the desired kinetic response.
Diffusion
The above partition coefficients are used to calculate the diffusion of a drug across the tissue-vascular barrier. All current drugs in the engine use perfusion limited diffusion as found in [182] [169]. For details on perfusion-limited and other diffusion modalities, see the Tissue Methodology.
In the current version of the engine, all drugs diffuse by the perfusion-limited model described above. An alternative means for modeling drug kinetics would be to leverage the other diffusion methods described in the Tissue Methodology report. The other diffusion models were developed primarily for the transmembrane transport of ions and a few specific organic molecules (e.g. glucose), and they have not been tested for drugs.
Clearance
The PBPK model represents renal, hepatic, and systemic clearance. The renal clearance rate, intrinsic clearance rate, and the systemic clearance rate are specified in the substance file in units of milliliter-blood per second per kilogram. The intrinsic clearance rate is used to calculate the hepatic clearance, as shown in Equation 3.
![]()
Where *ClH* is the hepatic clearance, *fu* is the fraction of the drug unbound in plasma, *ClI* is the intrinsic clearance, and BW is the patient body weight.
The amount of drug removed (cleared) from the system is found by calculating the amount of fluid volume that can be cleared by a healthy organ. The volume is calculated, as shown in Equation 4. This process is completed for the renal and hepatic clearance.
![]()
Where *Vcl* is the volume cleared, *ClH* is the organ clearance rate, BW is the body weight, and dt is the time step. The actual mass of the substance removed during this process is found by determining the amount of the drug in the volume cleared, as shown in Equation 5.
![]()
Where *Mcl* is the mass cleared, *Vcl* is the volume cleared, and C is the concentration in the tissue. The systemic clearance represents the total clearance for the body, including the renal and hepatic clearance. Therefore, the total volume cleared is calculated, then the renal and hepatic clearance volumes are removed, as shown in Equation 6. The “remaining” systemic mass cleared is calculated as shown in Equation 5. Half of the mass cleared from renal clearance is removed from each of the kidney tissue compartments, the mass cleared from hepatic clearance is removed from the liver tissue compartment, and the mass removed from the “remaining” systemic clearance is removed from the vena cava compartment. This is assumed to be from various metabolic processes in the plasma.
![]()
Where *Vcl* is the remaining systemic volume cleared, *Cls* is the systemic clearance rate, BW is the patient body weight, dt is the time step, *VclR* is the renal volume cleared, and *VclH* is the hepatic volume cleared.
Pharmacodynamics
Pharmacodynamic (PD) models characterize the effect that a drug has on the organism. The current version of the code contains two pharmacodynamic models.
- A simple dose-response model with a response biomarker functionally linked to the plasma concentration of a drug
- A site-of-action model with a local response functionally linked to the local tissue concentration of a drug
Plasma Concentration Model
Due to difficulties in measuring drug concentrations at the site of action as well as the varied and complex nature of the chain of events from receptor binding to biological response, drug responses are often quantified indirectly through surrogate endpoints. Development of the pharmaocodynamic model began with a characterization of expected results by subject matter experts for a small library of drugs. The expected results were limited to whole-body biomarkers similar to vital signs (e.g. changes in heart rate, respiration rate, blood pressure, etc.). We treated the expected results as surrogate endpoints and used the common Emax model to characterize the effects. Note that in this initial application, Emax referred to the expected effect of the drug rather than the maximum effect of the drug. For that reason, overdose is not possible with most of the drugs in the current version of the engine. However, the simple Emax model has been extended to a sigmoidal Emax model; therefore, it is possible to shape the response curve to better match empirical data, and setting the true maximum effect is as simple as setting the correct value in the drug file. We are beginning to convert the pharmacodynamics of some drugs to the true Emax model as data becomes available to us (see the future work section). The pharmacodynamic effects included in the plasma concentration PD model are described in Table 4.
| Pharmacodynamic Effect | Description | Available Values |
|---|---|---|
| Bronchodilation | Drug effect on the bronchii radii. A maximal effect of 1.0 will reverse the constriction of an asthma attack. | 0 to 1 |
| Diastolic Pressure | Change in the diastolic pressure. Given as a fraction. | -1 to 1 |
| Heart Rate | Change in the heart rate. Given as a fraction. | -1 to 1 |
| Neuromuscular Block | Drug’s strength as a neuromuscular block, with a 1.0 as the strongest block. | 0 to 1 |
| Pupil Size Modifier | Drug effect on the pupil diameter. Given as a modifier. | -1 to 1 |
| Pupil Reactivity Modifier | Drug effect on the pupil reactivity to light. Given as a modifier. | -1 to 1 |
| Respiration Rate | Change in the respiration rate. Given as a fraction. | -1 to 1 |
| Sedation | Drug’s strength as a sedative, with a 1.0 as the strongest sedative. | 0 to 1 |
| Systolic Pressure | Change in the systolic pressure. Given as a fraction. | -1 to 1 |
| Tidal Volume | Change in the tidal volume. Given as a fraction. | -1 to 1 |
| Tubular Permeability | Drug localized effect on tubulear permeability. Given as a severity, 1.0 complete reabsorption block | 0 to 1 |
The drug effects are specified for each drug in the substance file. The level of effect is calculated based on the expected effect (or, for some drugs, the maximum effect) of the drug and the current plasma concentration for the drug, as shown in Equation 7 [299].
![]()
Where *Em* is the expected (or maximum) effect of the drug, *EC50* is the concentration at 50% of the effect, *Cp* is the drug concentration in plasma, *Ebl* is the baseline for that effect (i.e., heart rate baseline), ΔE is the calculated effect of the drug, and η is the slope factor [299]. This calculation is repeated for each of the effects in Table 4.
The drug effect is applied as a fraction of the baseline for the biomarker, which is a patient variable in the engine. For example, a person with a resting heart rate of 72 bpm may have a physiological process happening during simulation, perhaps as a condition, which increases his/her baseline heart rate to 80 bpm. If this patient is given a drug with a heart rate effect of 0.2, then at maximum the patient's heart rate will be 96 bpm (80 + 0.2 * 80). If the same drug were given to the same patient but without the condition, the maximum heart rate will be 86.4 bpm (72 + 0.2 * 72).
The *EC50* values were unknown for the majority of the drugs, so it was estimated from the maximum concentration of the drug at a standard adult dose, as shown in Equation 8.
![]()
Where *Cmax* is the maximum plasma concentration for the standard adult dose and *EC50* is the concentration at which 50% of the effect should be present.
Cardiovascular Effects
Equation 7 provides a straightforward calculation for the heart rate. However, to be applied in the cardiovascular system, the drug effects need to be translated to the effects on the mean arterial pressure and the pulse pressure. This is accomplished using Equations 9 and 10. These equations were developed using the relationship between diastolic and systolic pressure and the mean arterial pressure [148].
![]()
![]()
Where MAP is the mean arterial blood pressure, DBP is the diastolic blood pressure, and SBP is the systolic blood pressure. These changes to the heart rate, mean arterial pressure, and pulse pressure are then system outputs for the drug system. The changes are then applied in the Cardiovascular System. For more details on the implementation of the effects see the Cardiovascular Methodology.
Respiratory Effects
As with the Cardiovascular System, the respiratory and tidal volume effects are relatively straight forward to apply. The neuromuscular block and sedation levels are calculated to have an effect level between zero and one, where zero is no effect and one is a maximal effect. The detail of how these are implemented can be found in the Respiratory Methodology.
Site-of-Action Model
There are currently two drugs which utilize a localized PD effects model: Albuterol and Furosemide.
Respiratory Effects
Aerosolized substances have a direct effect on the respiratory system. There are two direct-effect aerosol substance properties.
- BronchioleModifier
- A parameter which controls the dilation (positive value) or constriction (negative value) of the bronchioles in the presence of the substance
- InflammationCoefficient
- A parameter to quantify the propensity of the aerosol to cause inflammation of the respiratory tissues
For example, Albuterol is an aerosolized drug that typically enters the body via nebulizer or metered-dose inhaler. Accordingly, in the engine Albuterol enters the body through the respiratory tract and diffuses into the respiratory tissues (see the aerosol section in the Environment Methodology report). The BronchioleModifier for Albuterol is set to 1 in the substance file, meaning that Albuterol has the capability to maximally dilate the bronchioles. The bronchodilatory effects of Albuterol are applied directly at the site of action, and the actual bronchodilation is a function of the concentration of Albuterol in the respiratory tissues.
Renal Effects
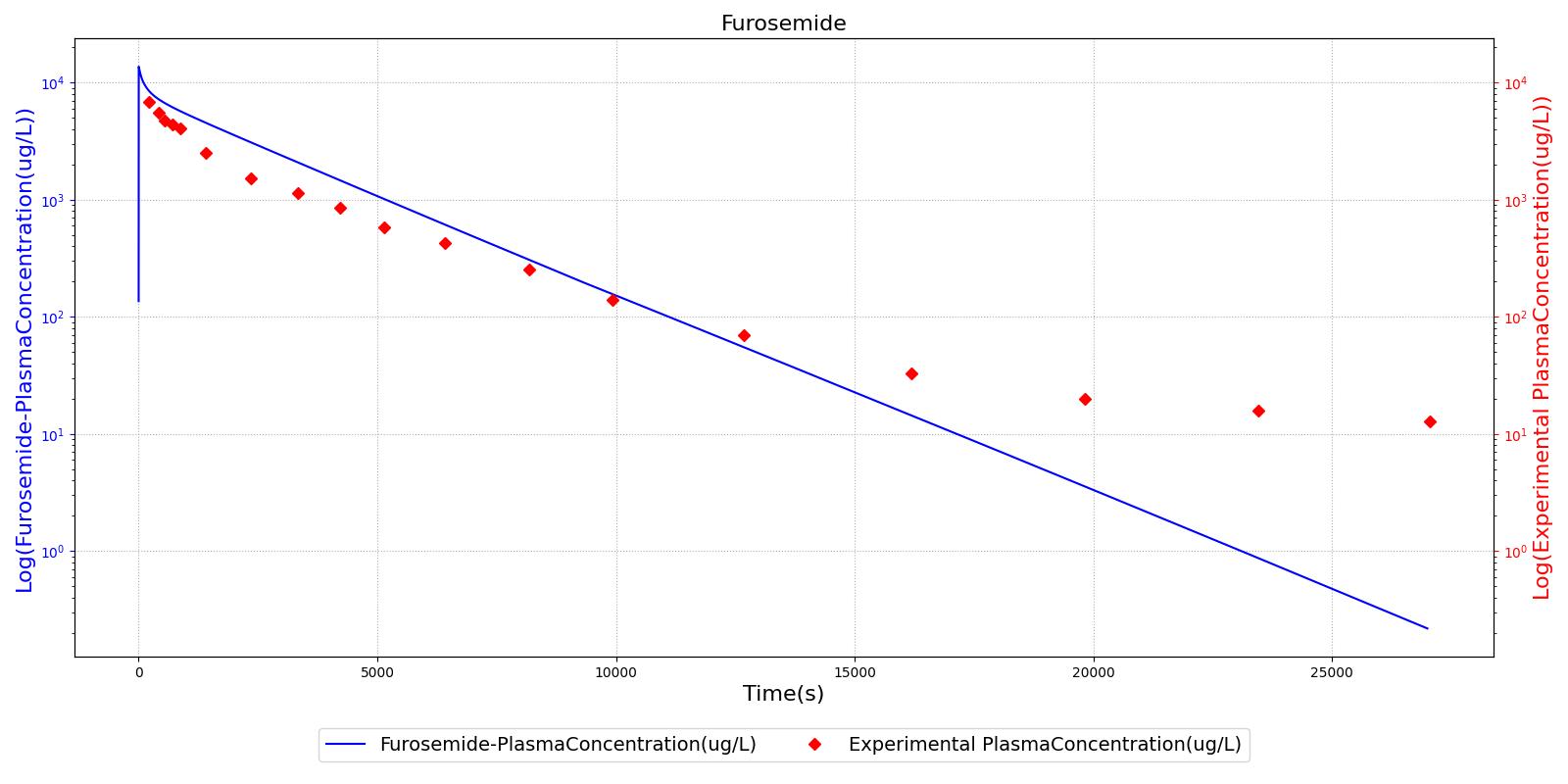
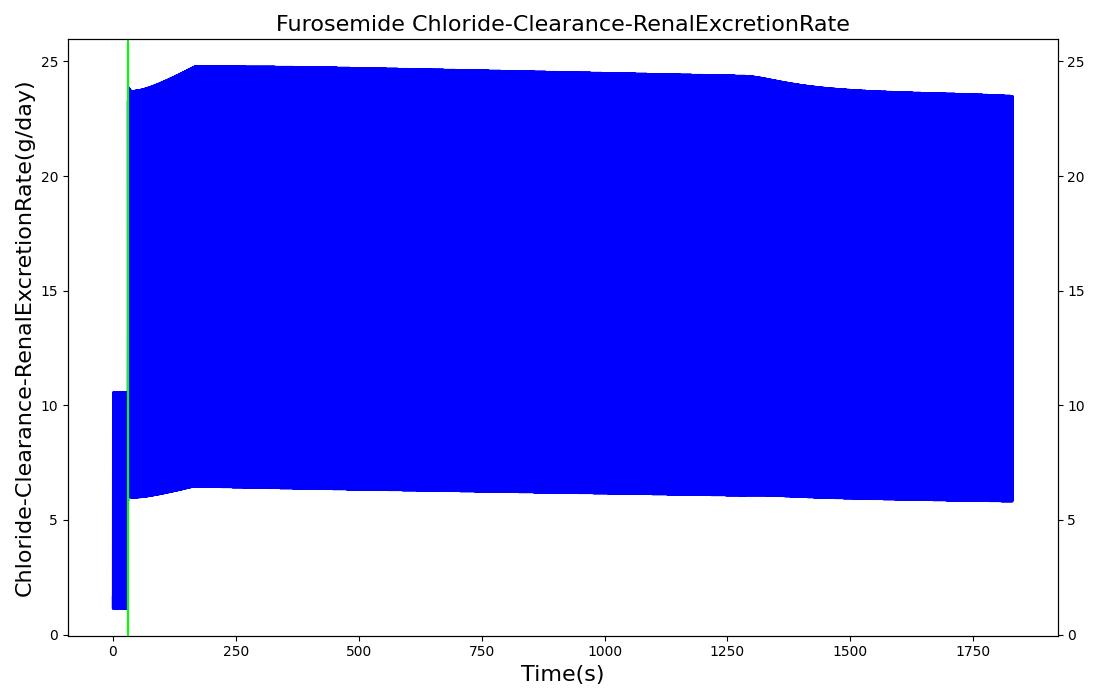
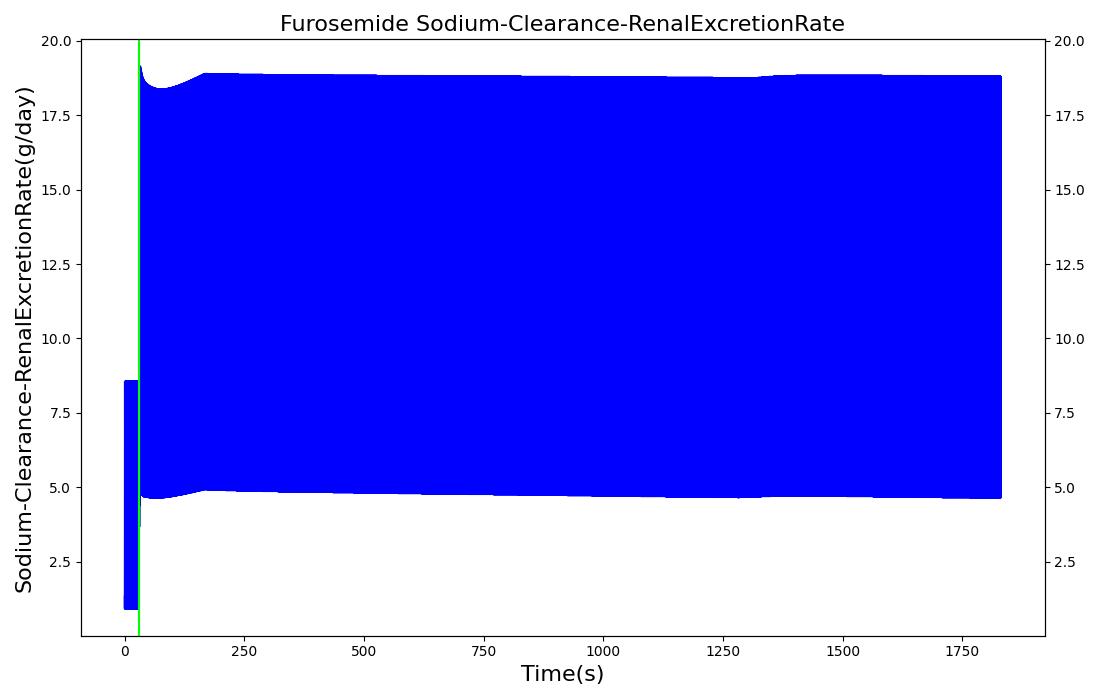
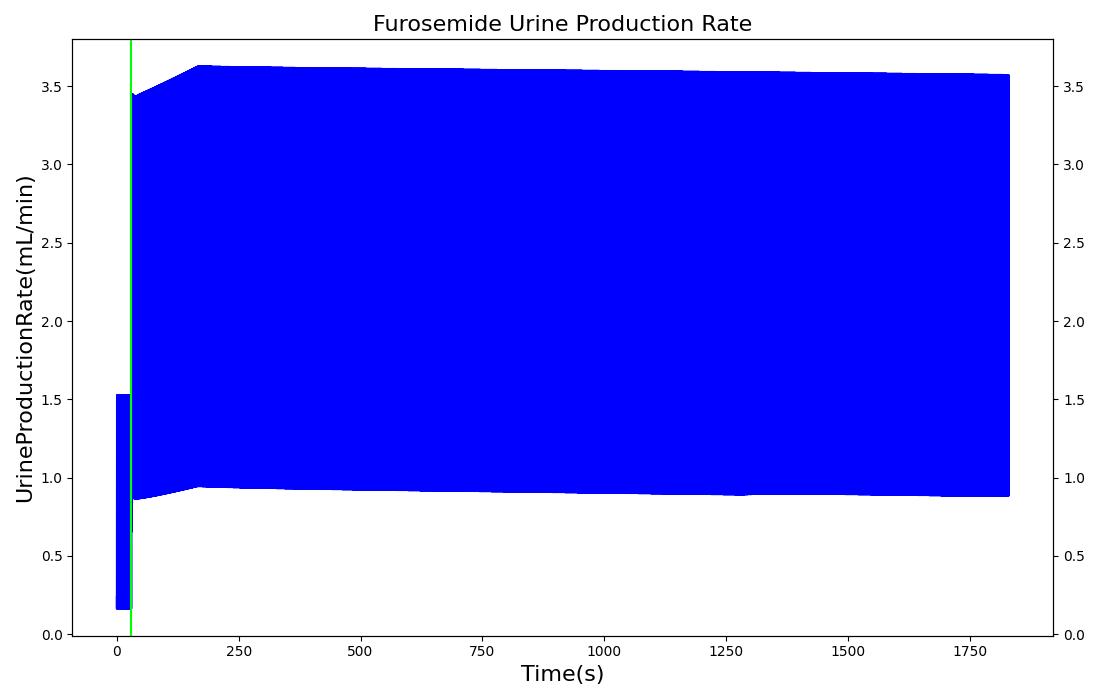
The pharmacodynamic effects of diuretics locally target the renal system's functionality. Local binding of the Na-K-2Cl symporter by Furosemide (Lasix) in the tubular lumen stimulates an increase in urine production and ion excretion. In the engine we simulate this effect by adjusting the reabsorption permeability as a function of plasma concentration of Furosemide. This enables simulation of the whole-body drug effects, but the model does not use the local concentration of drug in the tubular lumen as the primary determinant of the reabsorption adjustment. A mechanistic model based on traditional receptor theory which combines the tissue properties of receptor concentration and signal transduction with the drug properties of intrinsic efficacy and affinity to form a model of drug response would use the local tissue concentration as the first link in a causal chain leading to permeabilty changes and the appropriate downstream effects. This type of mechanistic model is recommended as future work. For details on the reabsorption and urine production of the renal system, see the Renal Methodology report. The results of the Lasix administration simulation are compared to data found in literature and presented in the validation section.
Substances
The full list of drugs available in the data library can be found in Table 5 in the Validation section. Additional substances, such as oxygen, carbon dioxide, and hemoglobin that are available in the engine can be found in the Blood Chemistry Methodology.
Patient Variability
It is possible to customize the body that is simulated. For a detailed discussion of patient variability please see the Patient Methodology report. Variability in body morphology will obviously have an effect on the pharmacokinetics of a drug. For example, a larger body may have a larger plasma volume, and thus the plasma concentration for a specific dose of a drug will be less than the same dose administered to a smaller body. Additionally, the partitioning of drugs is dependent on the relative masses of the physiological compartments, and storage is affected by distribution. Lipophilic drugs may appear to remain longer in the engine plasma in patients who are configured with a higher body fat fraction. Patient configuration will also impact the pharmacodynamics. Maximum drug effects are computed as a fraction of baseline physiology. Changing a patient's baseline parameter (e.g. heart rate baseline) will also change the maximum effect (e.g. maximum heart rate) at a specific concentration of a drug. All drug validation is performed on a Standard Male.
Dependencies
The substance calculations rely on the flow values calculated by the Circuit Methodology to calculate the movement of substances from compartment to compartment in each circuit. Any limitations and assumptions associated with this methodology will also be associated with the Drugs Methodology. The pharmocokinetic modeling is reliant on accurate values for the physical chemical properties of drugs. If these parameters are unknown or estimated from animal studies, the calculation for the partition coefficient may become unreliable. For some drugs, this information may be difficult to determine. The model also allows the user to supply partition coefficients directly. Some third party resources, both open source and commercially available, may provide this data for users, allowing incorporation of the data into the full body simulation.
While this model is primarily used for drugs, it could be used for any substance in the body. The engine uses the clearance methodology for several additional substances. The modular approach to these calculations allows for the use of any combination of the three methodologies, PK, clearance, and PD.
The substance values and calculations are used by a variety of systems to trigger actions and responses, define physiologic set points, scale circuit parameters, and modify driver frequencies. Many of these triggers and responses are discussed in the Blood Chemistry Methodology and the Endocrine Methodology. The drug-specific effects are listed and described briefly in Table 5. The implementation of these calculated responses can be found in the Cardiovascular Methodology and the Respiratory Methodology.
Assumptions and Limitations
The Drugs Methodology is based on the assumption that all substances are suspended in the fluid (blood or air, depending on the system) and move through the circuits with the flow. While this does allow for renal and hepatic clearance and metabolic elimination of substances, it does not allow for particle deposition. This assumption is valid for the level of fidelity of the model. In the future, particle deposition can be addressed by adding a more detailed substance methodology with factors for deposition within an organ, tissue, or blood vessel. Also, not included is a method for modeling vaporized substances, such as albuterol. In the future, we will add this methodology to allow a liquid in the respiratory tract and produce a more realistic representation of the behavior.
The drug model addresses many of the earlier limitation of the drug model. This includes creating a substance file that uses physical chemical properties to define the pharmacokinetic properties and intuitive modifiers to calculate the pharmacodynamic effects. However, this implementation of a PD model works best with a drug with immediate effects, such as anesthesia medications. This implementation has not been tested for drugs with a later onset due to secondary mechanisms. This is an area for future work. Additionally, no drug interactions or intoxication is modeled.
We have recently added an "oversedation" substance. This uses the PK/PD models, but is an unvalidated, rudimentary implementation of the respiratory distress and eventual respiratory failure associated with oversedation or some forms of intoxication. We will be revisiting this model in the future. However, a user can administer the "oversedation" substance to trigger the associated respiratory distress. We tested with a 50 ug dose.
Actions
Interventions
Substance Bolus
A bolus administration represents an injection of a substance in a single dose. The substance, concentration, and amount are specified by the user. The total mass for the dose is calculated by multiplying the concentration and the amount. The total mass is then incrementally added to the vena cava compartment over the course of two seconds to represent the slow depression of a plunger. Once added to the vena cava, the substance will circulate through the Cardiovascular System as part of the blood flow. The effects of the substance on the physiologic output will vary with the substance.
Substance IV Infusion
Titration represents the administration of a single substance at a specified rate and concentration over time into the venous system. The user selects a single substance and specifies the concentration and rate with titration. As with intravenous administration, the mass of the substance is incremented based on the amount of the substance entering the system. The mass is calculated by taking the flow entering the system at each time step multiplied by the known concentration of each substance within the compound substance. This mass is then added to the vena cava at each time step until the intravenous administration is stopped. The added substances then circulate through the Cardiovascular System as part of the blood flow. The effect on the physiologic output will vary based on the compound substance selected.
Compound IV Infusion
Intravenous administration represents administration of a compound substance (a single entity with multiple substances, i.e., saline) at a specified rate over time into the venous system. The compound substance, the rate, and the bag volume are specified by the user. At each time step, the mass of the substances are incremented based on the amount of compound substance entering the system. The mass is calculated by taking the flow entering the system at each time step multiplied by the known concentration of each substance within the compound substance. This mass is then added to the vena cava at each time step until the intravenous administration is stopped. The added substances will then circulate through the Cardiovascular System as part of the blood flow. The effect on the physiologic output will vary based on the compound substance selected.
Results and Conclusions
Validation - Resting Physiologic State
The Drugs Methodology has no resting state validation.
Validation - Actions
Bolus Administration
All drugs in the data library were validated qualitatively or with subject matter expert input. The bolus injection methodology was validated by comparing the PK and PD results to literature. See the sections below for more detail.
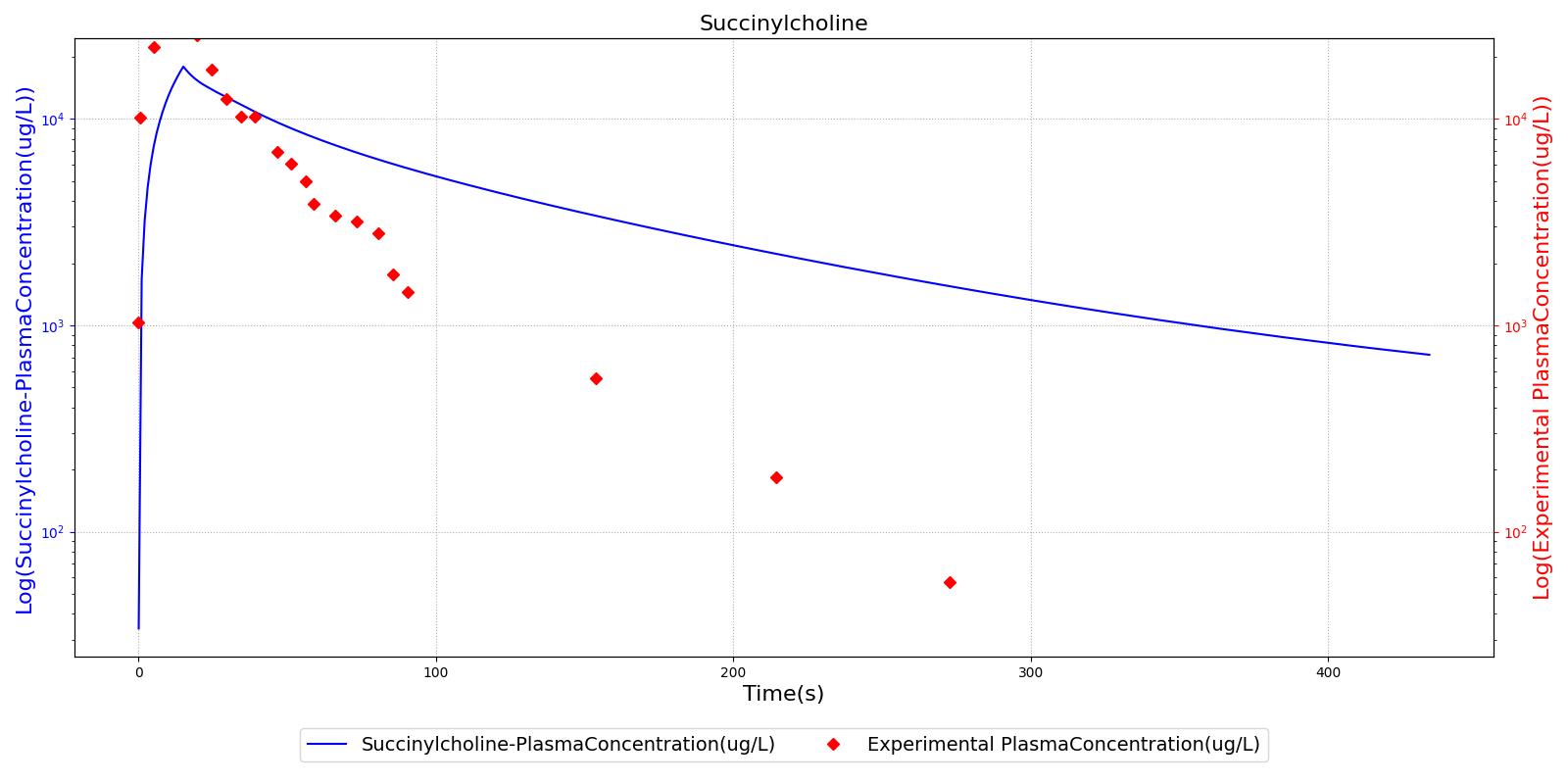
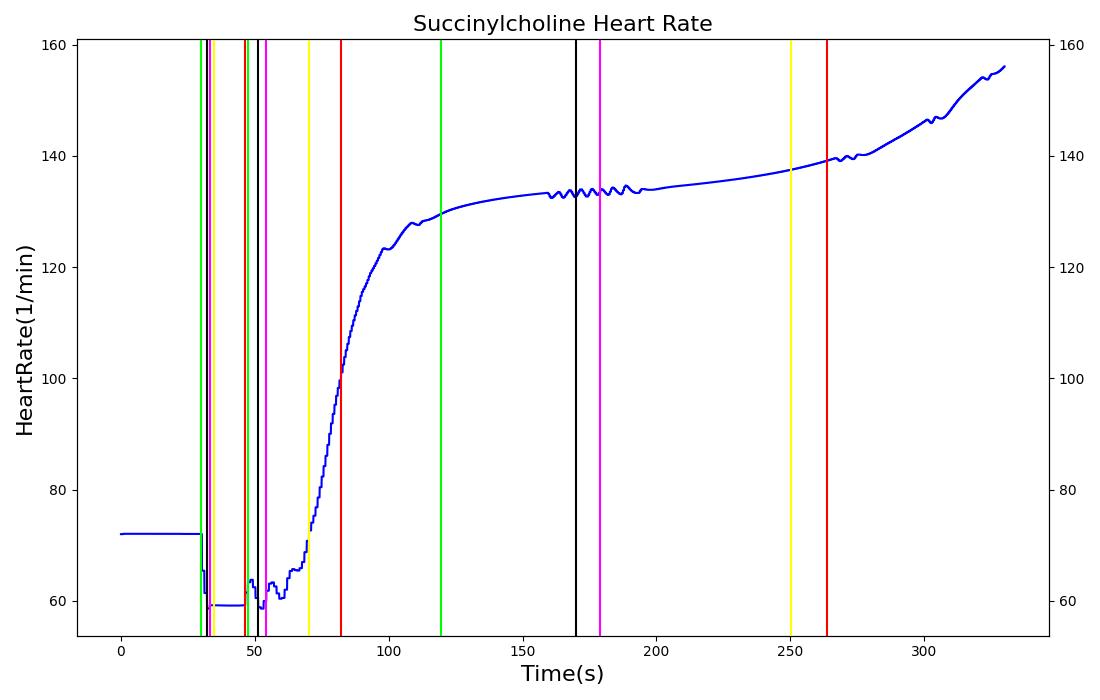
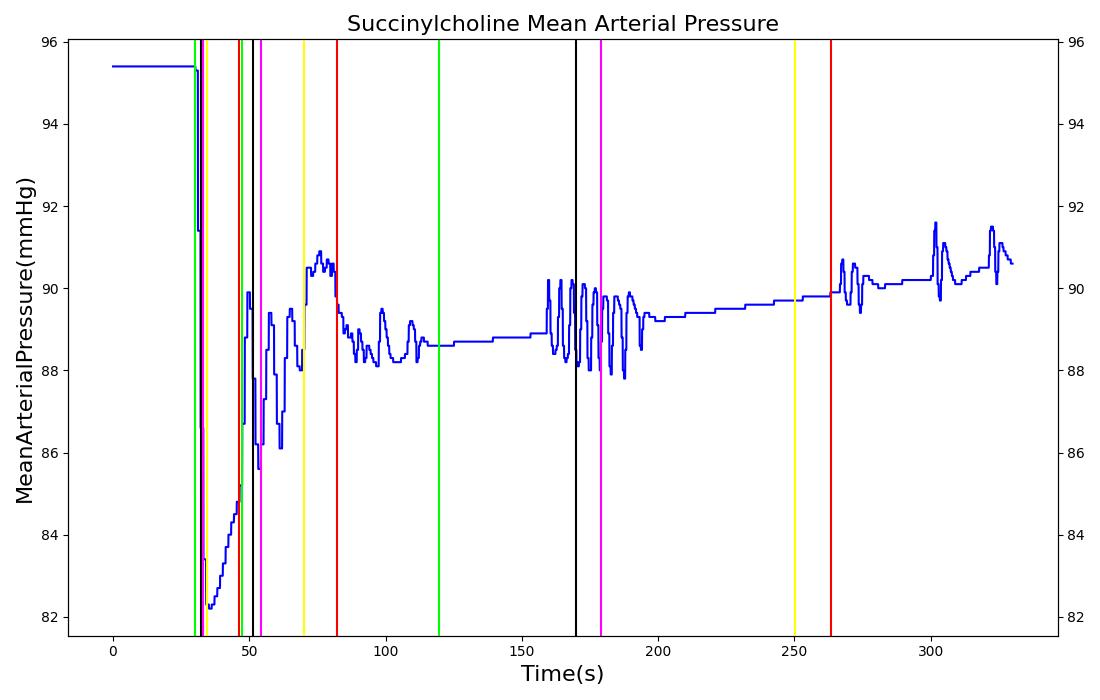
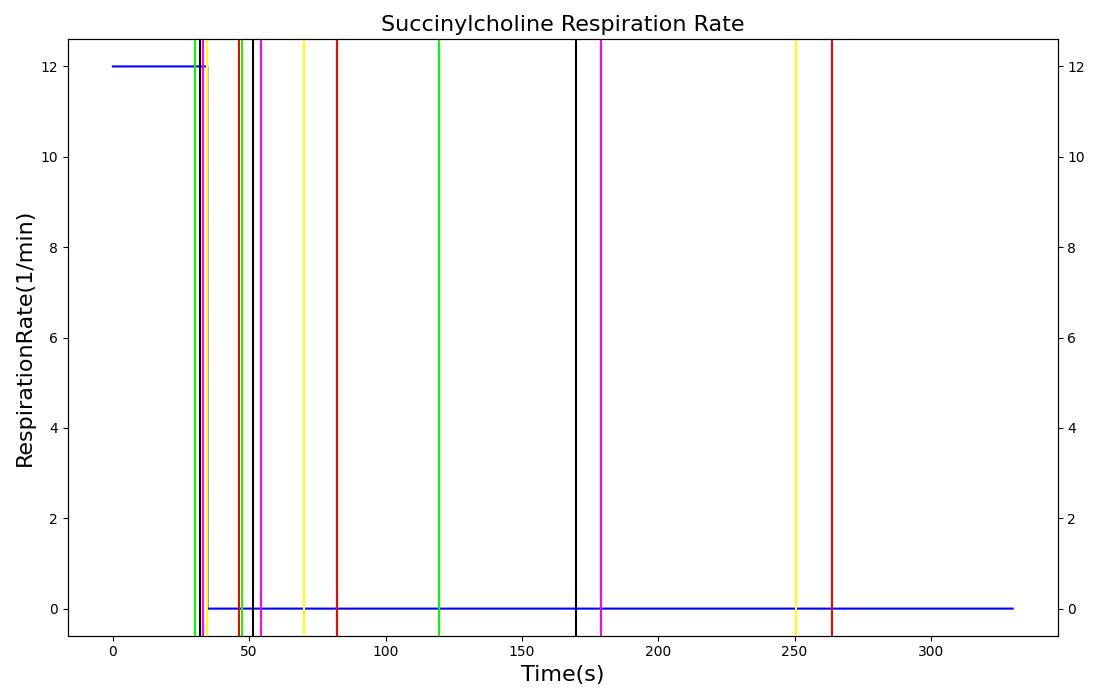
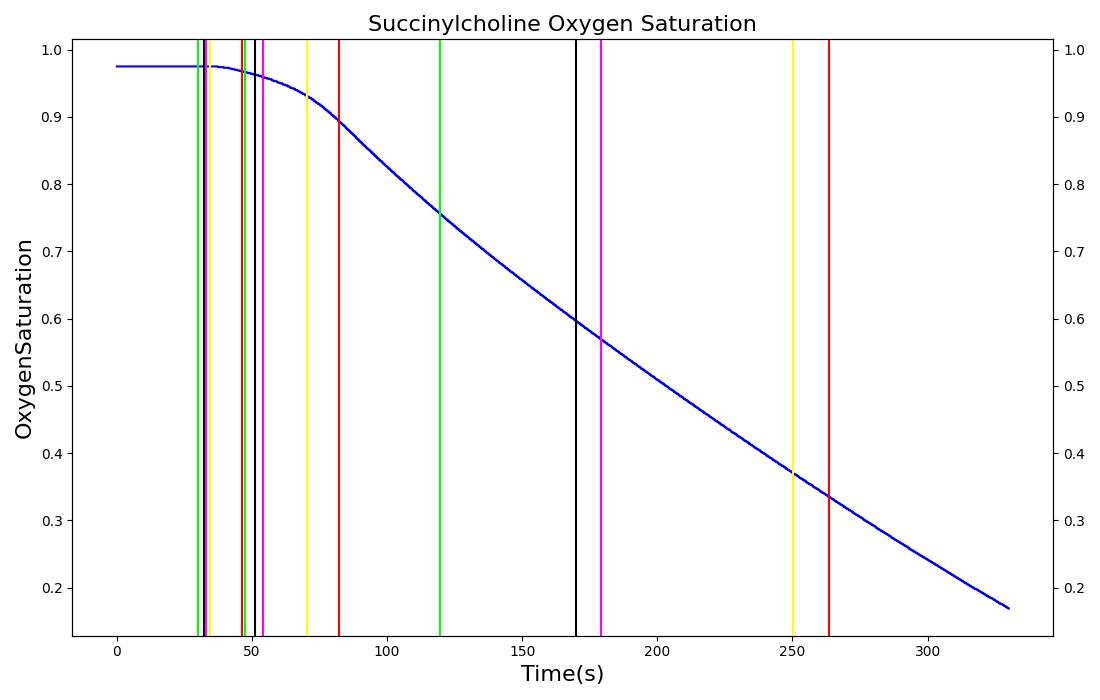
The succinycholine scenario shows a drug impacting the major engine systems. Succinycholine (Figure 1) initially drops the heart rate and blood pressure approximately 5-10%. As expected, the respiration drops to zero within 60-90 seconds to represent the paralysis associated with the neuromuscular blocker. The oxygen saturation drops slowly as the concentration of oxygen in the bloodstream drops, leading to hypoxia. Epinephrine is released in response to hypoxia, causing an increase in heart rate and blood pressure (Endocrine Methodology). This is an example of the interdependent nature of the engine systems and the chain reaction drug administration can cause.
 |  |
 |  |
 | |
Intravenous Administration
Validation of intravenous administration of a hypotonic solution and blood products can be found in the Cardiovascular Methodology.
Validation - Pharmacokinetic
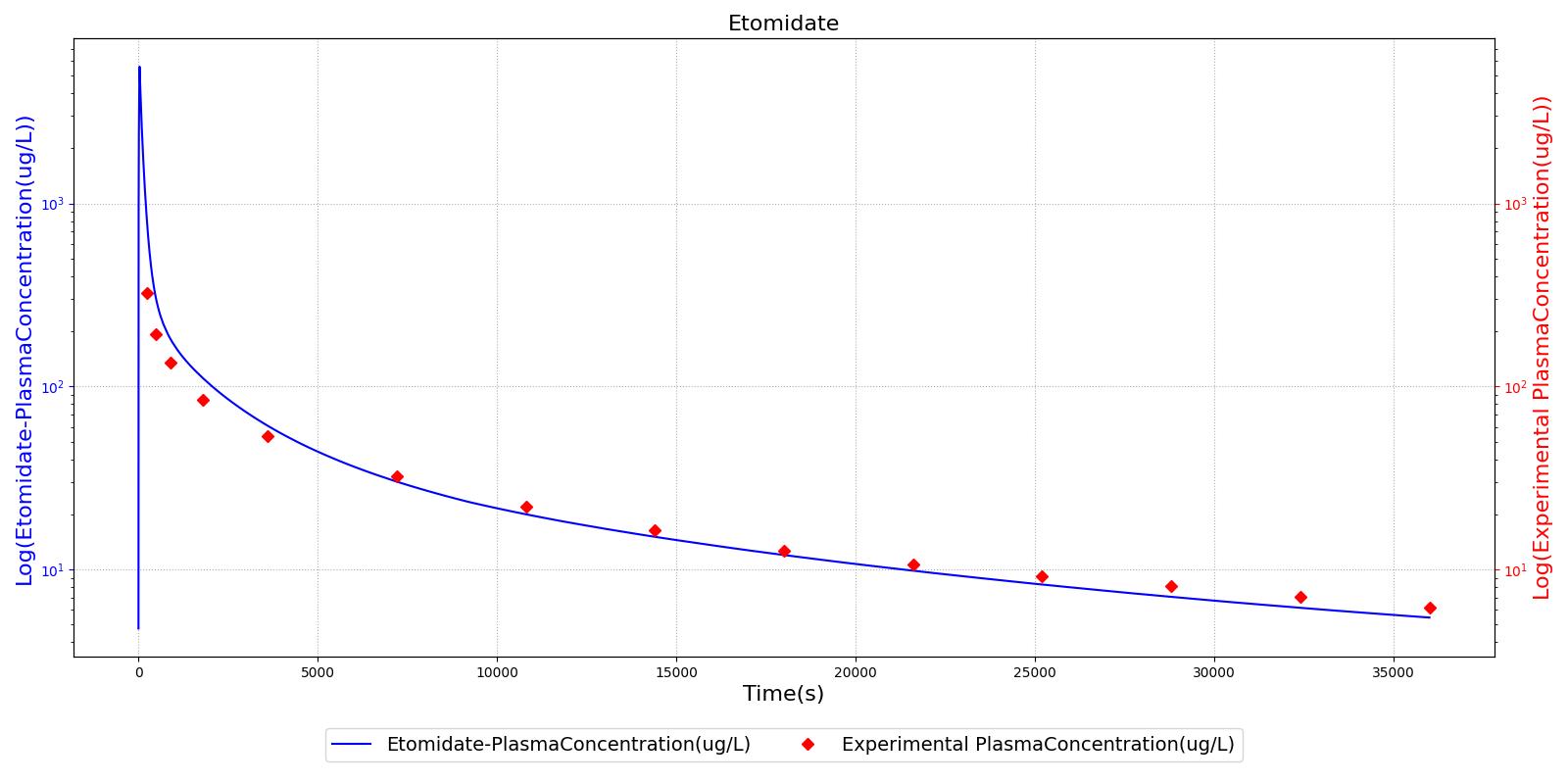
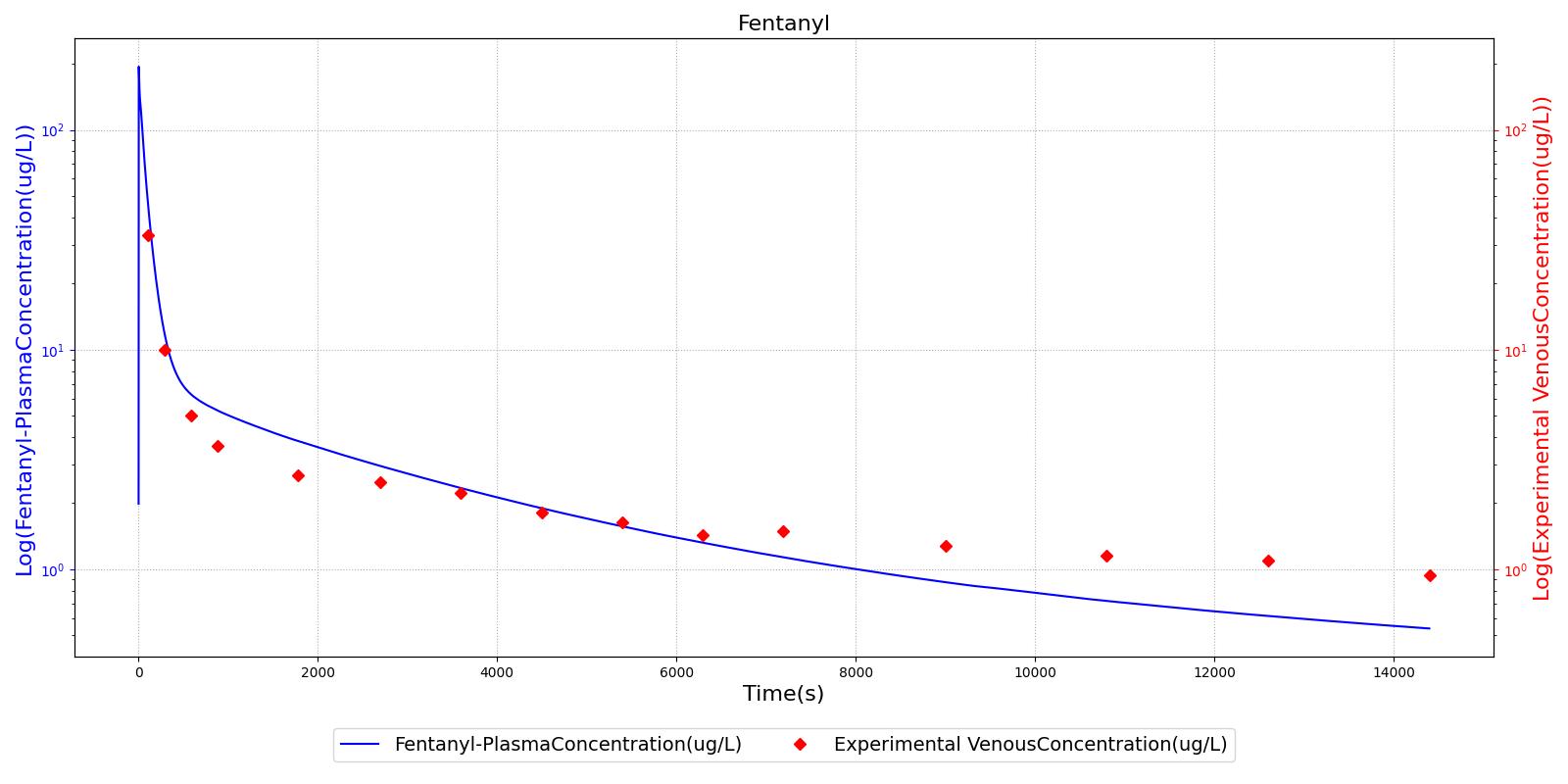
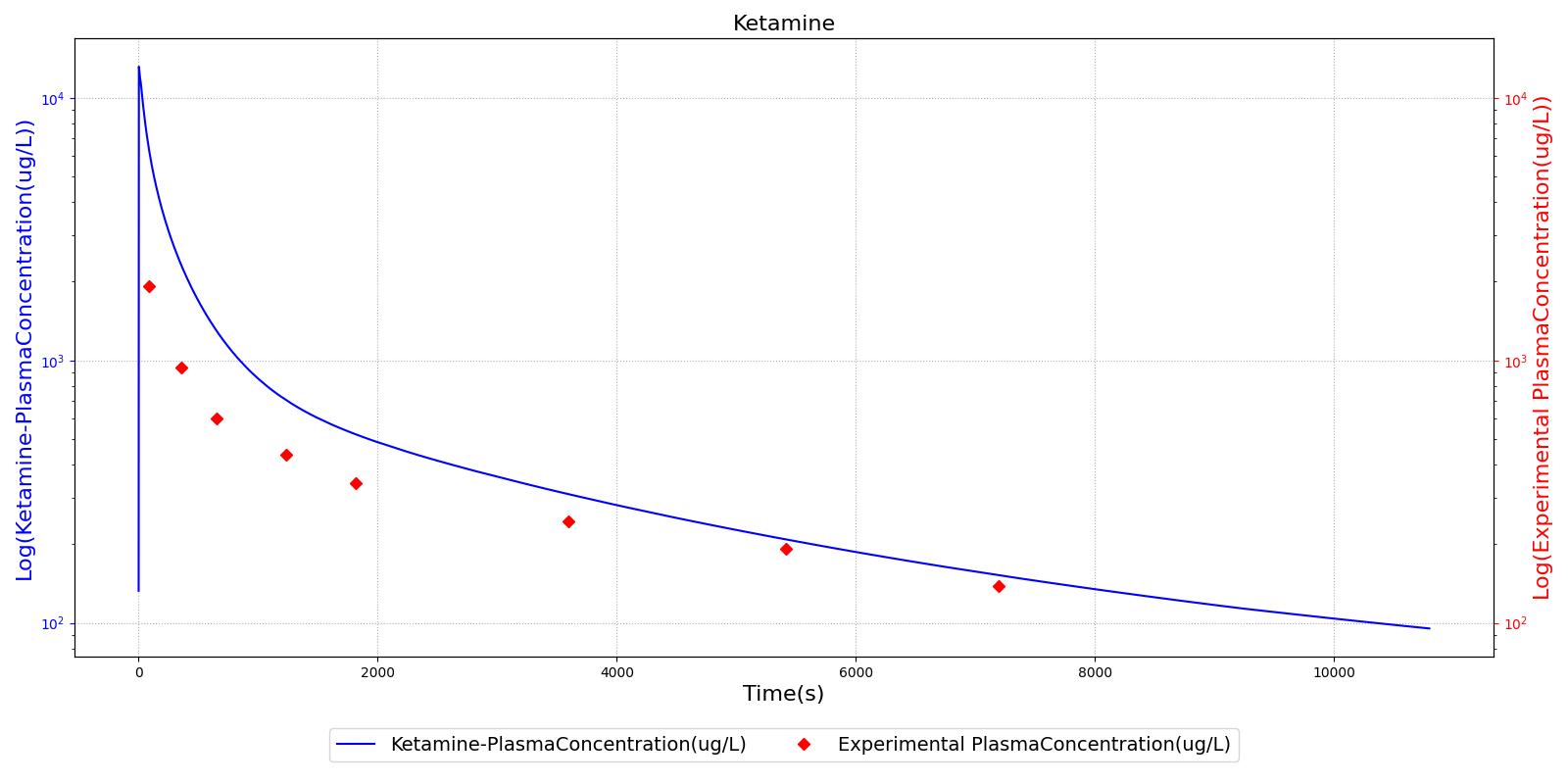
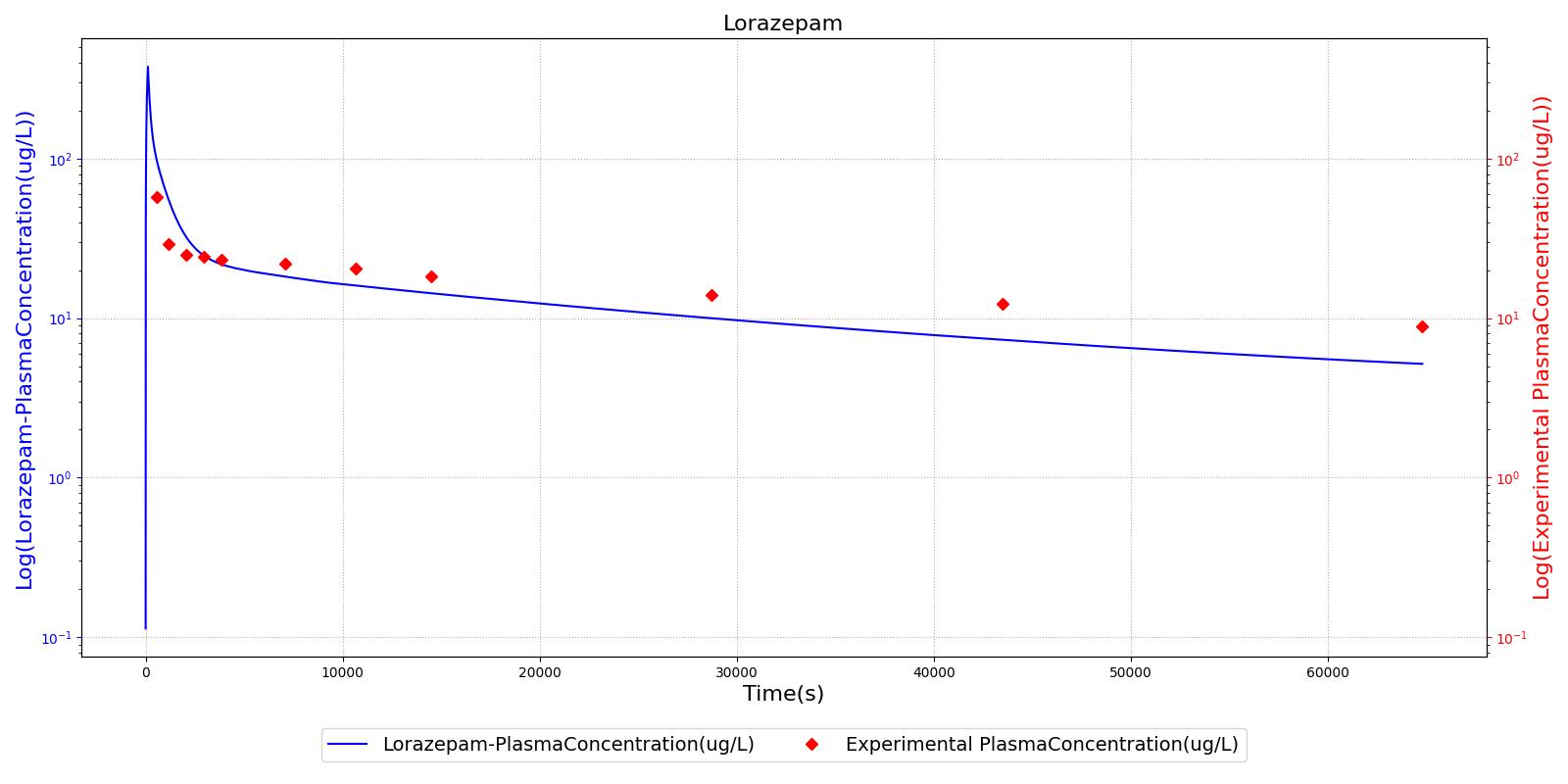
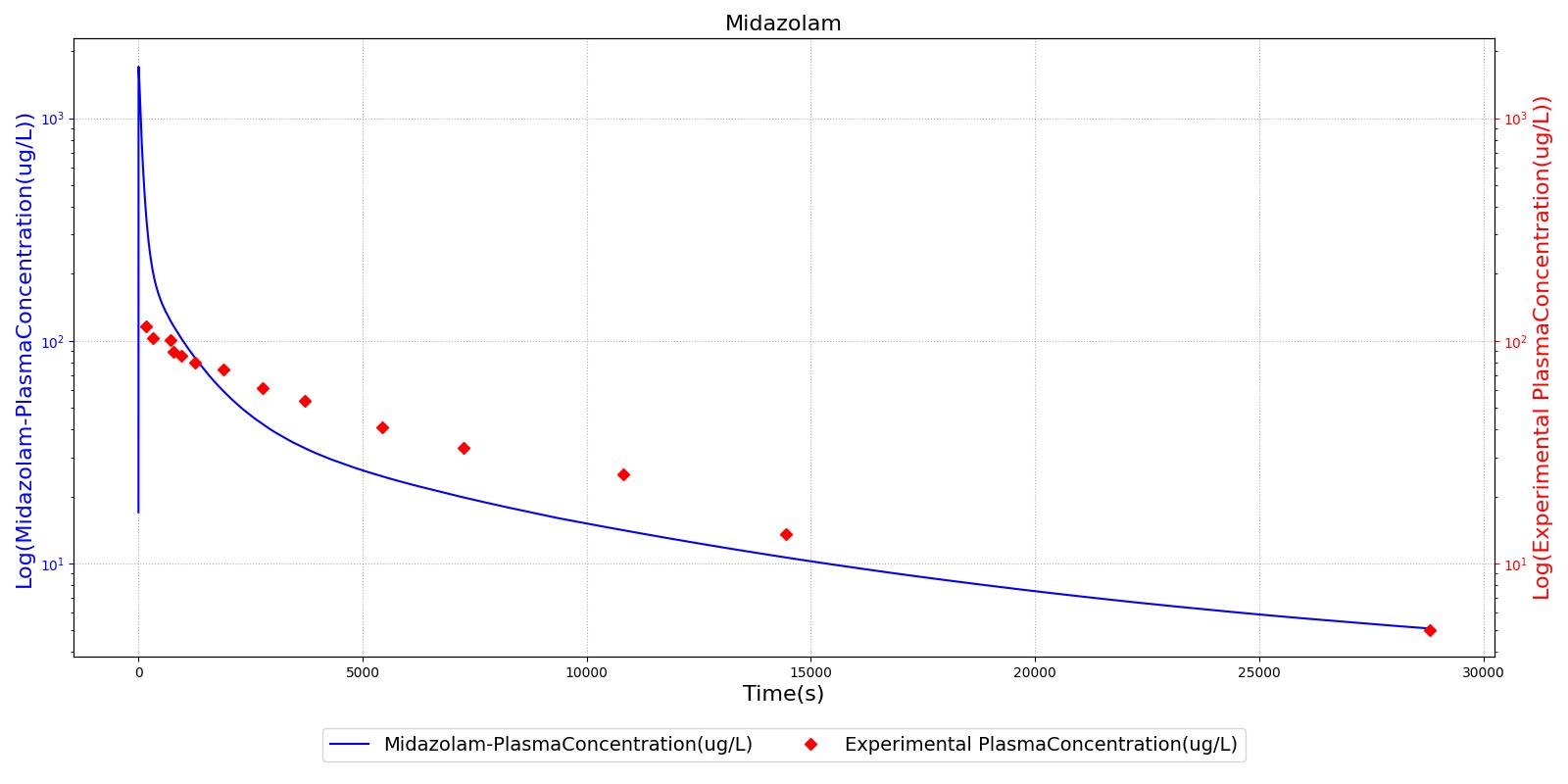
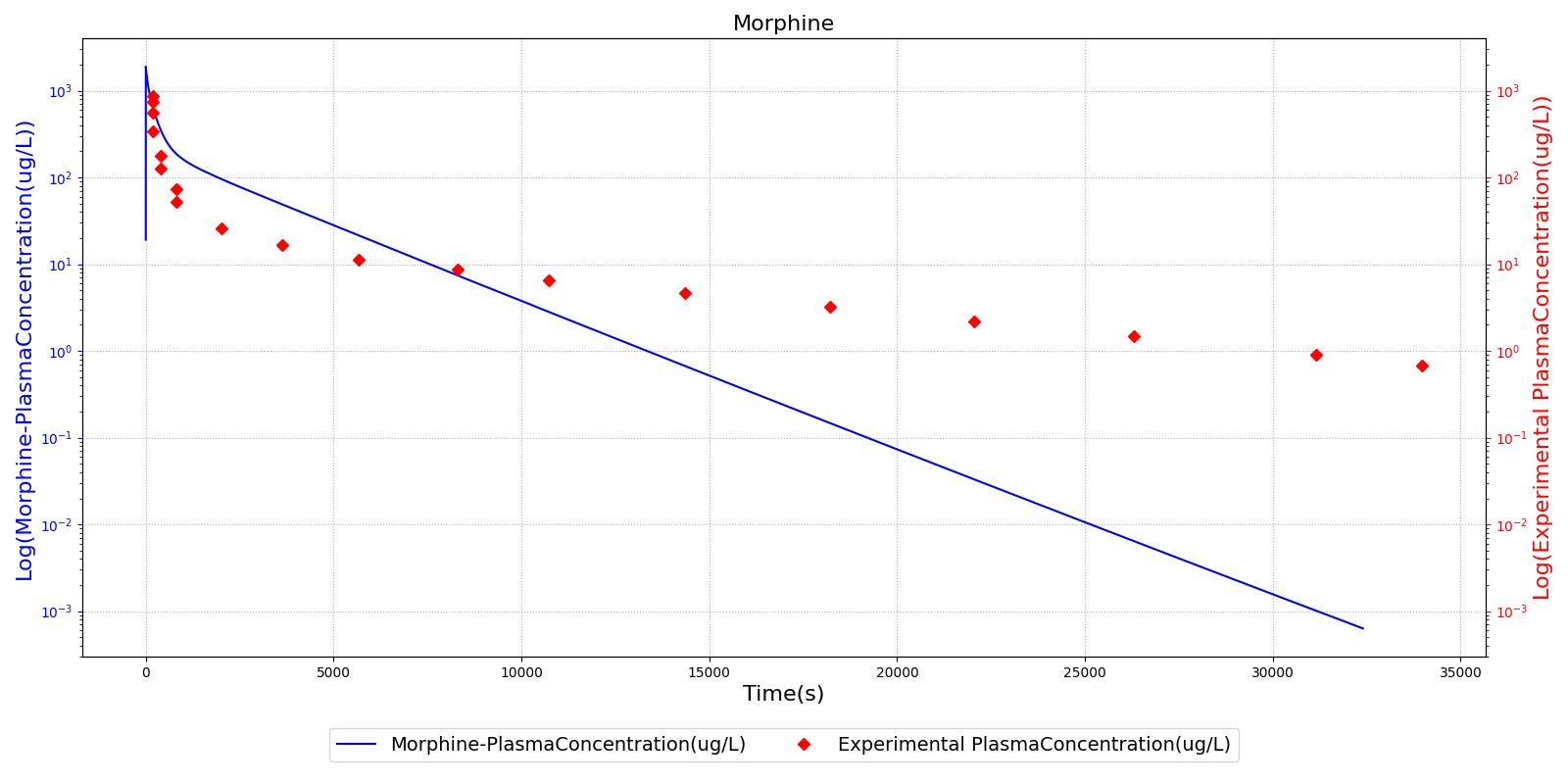
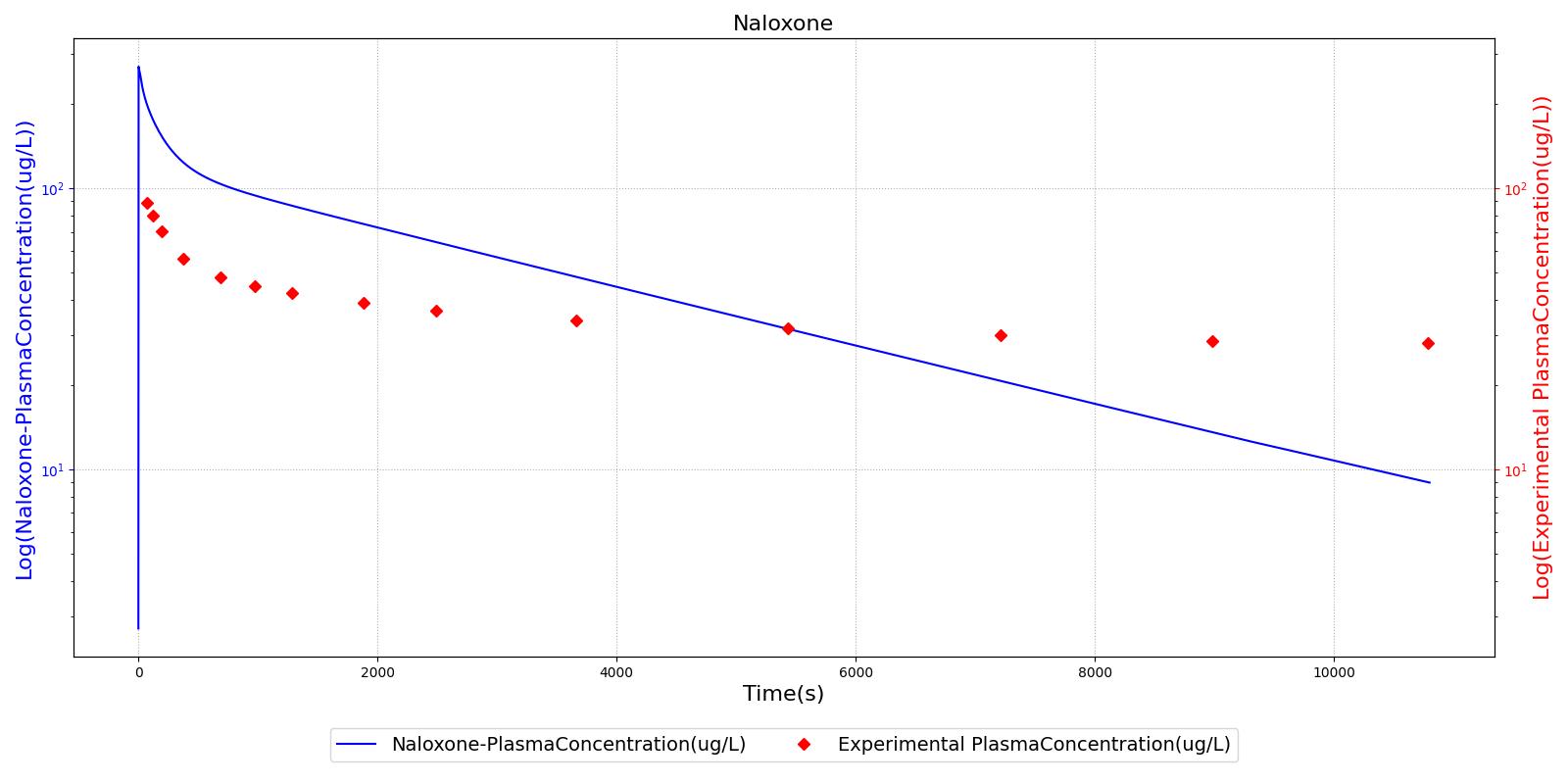
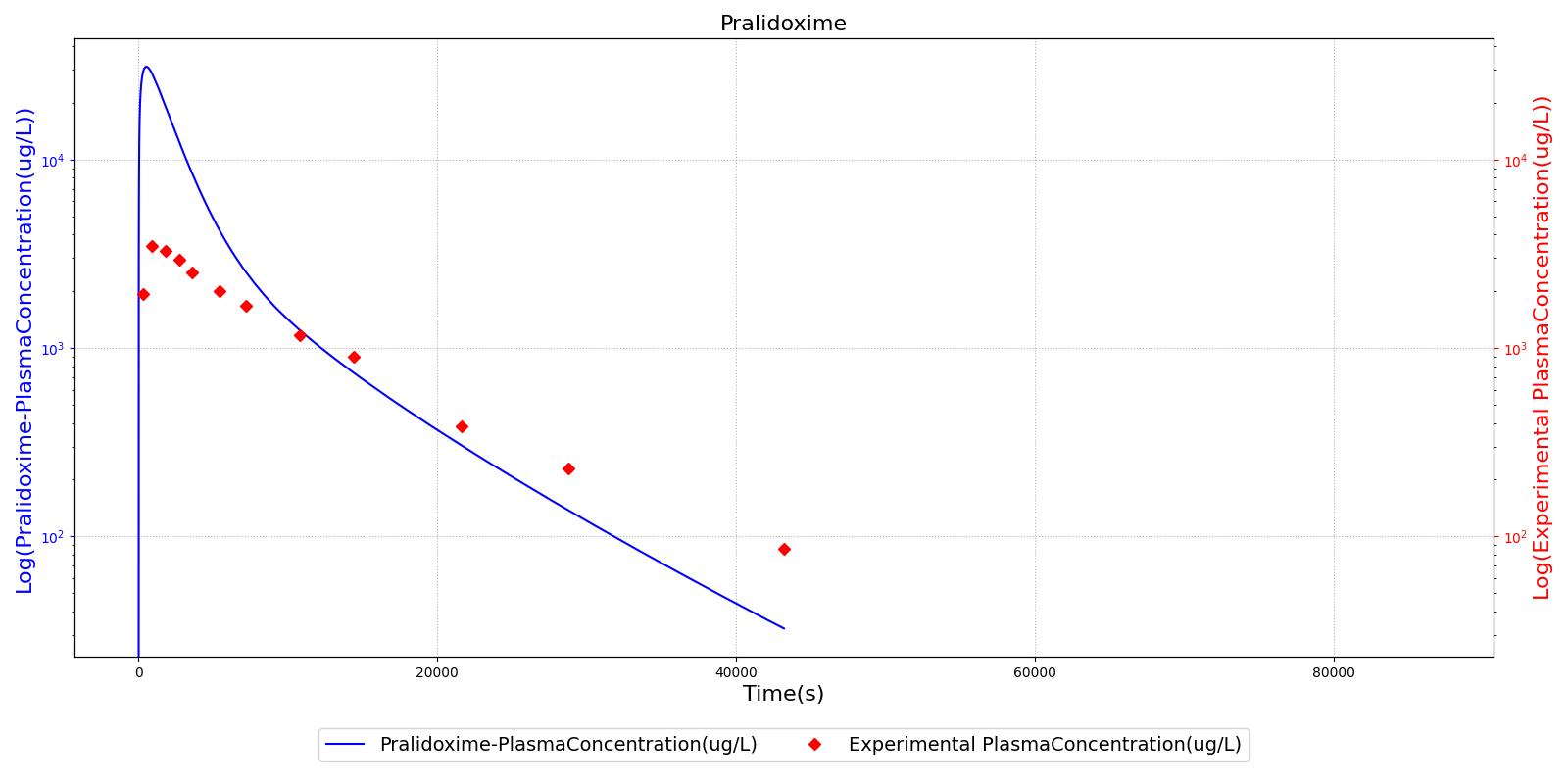
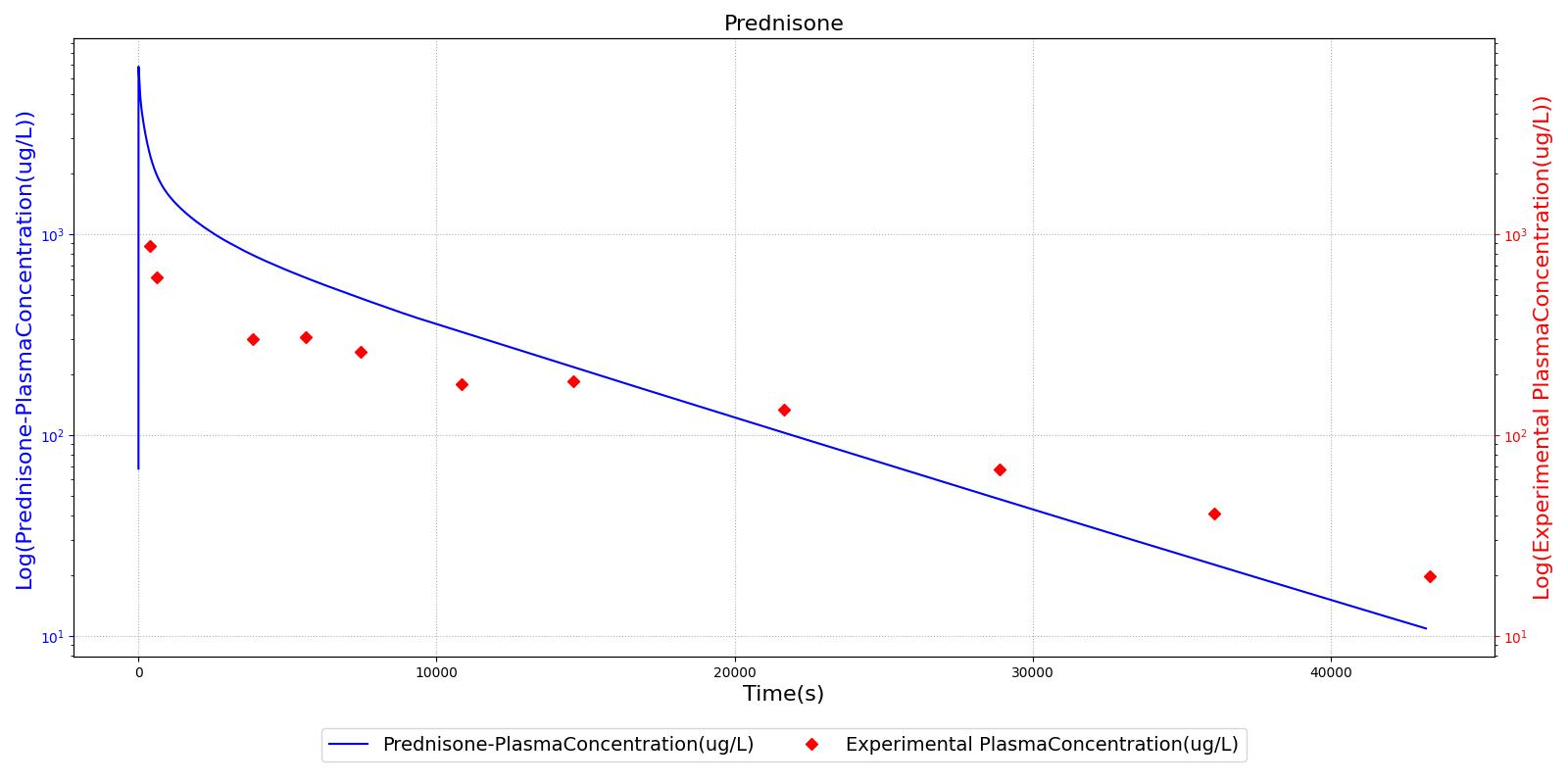
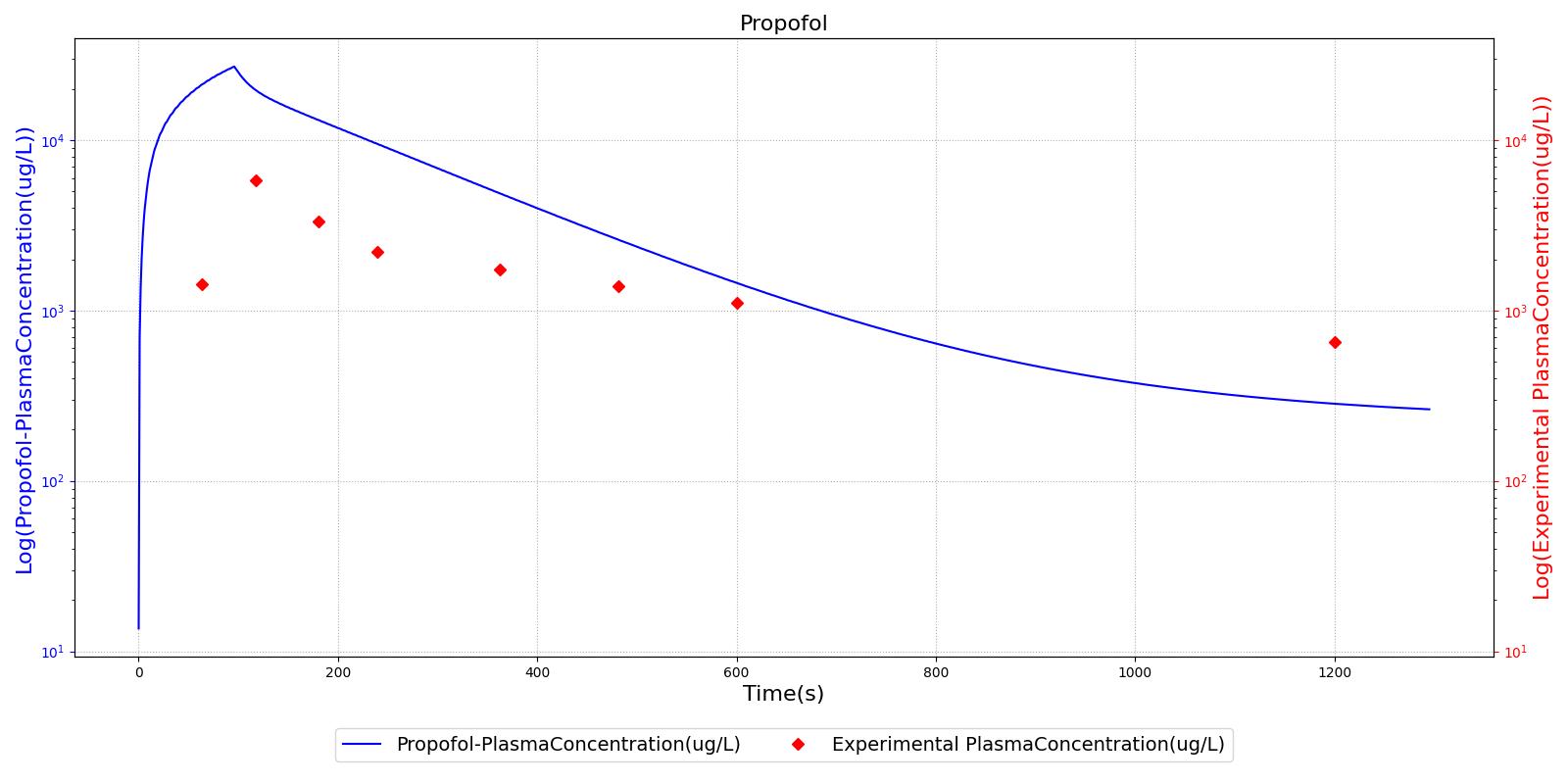
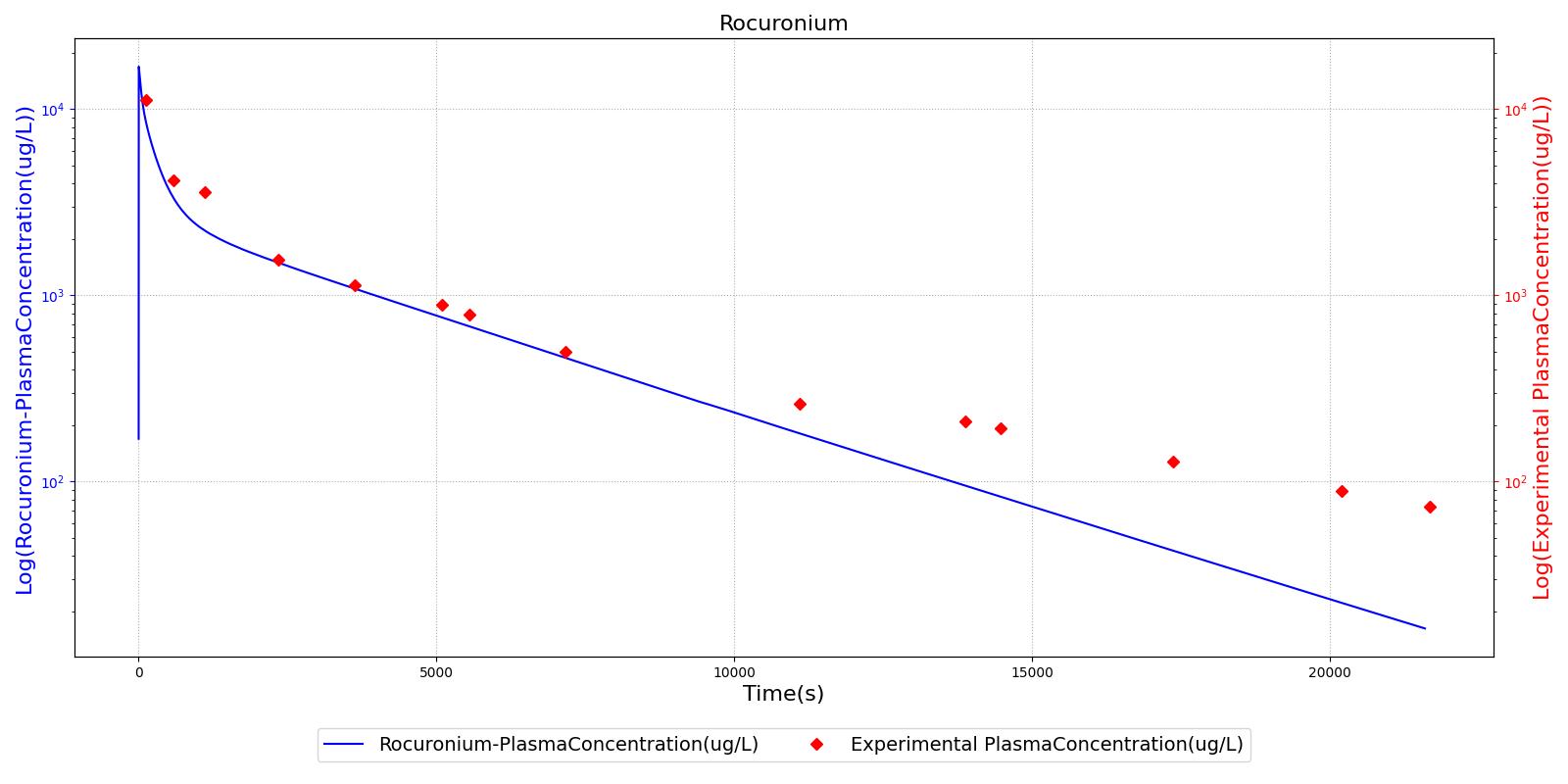
The PK model was validated by comparing the plasma concentration calculated by the engine to plasma concentration curves found in the literature. The comparisons are shown in Figures 2-14. No available data was found for Epinephrine, so this drug was only validated from a PD perspective.
The above results show that, in general, the model chosen for the engine produces good agreement between experimental and calculated data. However, it also reveals that for some drugs, this model does not appear to be valid. Future work will investigate the incorporation of vaporized substance methodologies, and additional partition coefficient calculations that may prove more accurate for specific drug types. Further statistical analysis will also be undertaken to assess the model performance rather than solely relying on a qualitative analysis.
Validation - Pharmacodynamic
The pharmacodynamic effects of the drugs were validated by comparing the effects of each drug to a number of clinical parameters. A summary of the drug validation is shown in Table 5. More details on the individual scenario validation can be found in the Drugs Scenario Validation Matrix.
| Scenario | Description | Good | Decent | Bad |
|---|---|---|---|---|
| Albuterol | Administer 90 ug metered dose of Albuterol | 5 | 0 | 0 |
| Etomidate | Administer 21 ug injection of Etomidate | 5 | 0 | 0 |
| Epinephrine | Administer 10 ug injection of Epinephrine | 4 | 1 | 0 |
| Fentanyl | Administer 17 mg injection of Fentanyl | 5 | 0 | 0 |
| Ketamine | Administer 115 mg injection of Ketamine | 4 | 1 | 0 |
| Lorazepam | Administer 2 mg injection of Lorazepam | 5 | 0 | 0 |
| Midazolam | Administer 5 mg injection of Midazolam | 5 | 0 | 0 |
| Morphine | Administer 42 mg injection of Morphine | 5 | 0 | 0 |
| Naloxone | Administer 17 mg injection of Fentanyl Followed by 30 ug injection of Naloxone *Validating only Naloxone | 5 | 0 | 0 |
| Pralidoxime | Administer 700 mg injection of Pralidoxime | 5 | 0 | 0 |
| Prednisone | Administer 20 mg injection of Prednisone | 5 | 0 | 0 |
| Propofol | Administer 135 mg injection of Propofol | 4 | 0 | 1 |
| Rocuronium | Administer 52 mg injection of Rocuronium | 2 | 0 | 3 |
| Succinylcholine | Administer 96 mg injection of Succinylcholine | 5 | 0 | 0 |
| Total | 64 | 2 | 4 |
| Event | Notes | Action Occurrence Time (s) | Sample Scenario Time (s) | Heart Rate (beats/min) | Systolic Pressure (mmHg) | Diastolic Pressure (mmHg) | Respiration Rate (breaths/min) | Oxygen Saturation |
|---|---|---|---|---|---|---|---|---|
| Administer Epinephrine - 10ug | Drug Onset < 1 minute | 30 | 150 | 25-50% Increase [253] | 25-50% Increase [253] | 25-50% Increase [253] | NC [253] | NC |
| Administer Etomidate - 21 mg | Drug Onset < 1 minute | 30 | 100 | Minimal Changes [362] | Minimal Changes [362] | Minimal Changes [362] | Little Respiratory Depression [362] | NC |
| Administer Fentanyl - 17 mg | Drug Onset < two minutes | 30 | 100 | Stable [126] p192-7, [265] p277; 5-10% Decrease [253] | Stable [126] p192-7, [265] p277; 5-10% Decrease [253] | Stable [126] p192-7, [265] p277; 5-10% Decrease [253] | Decrease [126] p192-7, [265] p277; 15-25% Decrease [253] | NC |
| Administer Ketamine - 115 mg | Drug Onset < 1 minute | 30 | 200 | Moderate Increase [126] p200; 15-25% Increase [253] | Moderate Increase [126] p200; 15-25% Increase [253] | Moderate Increase [126] p200; 15-25% Increase [253] | Mild Decrease [126] p200; 25-50% Decrease [253] | NC |
| Administer Lorazepam - 2 mg | Drug Onset < 2 minutes | 30 | 350 | Mild Decrease [348] | Mild Decrease [348] | Mild Decrease [348] | Mild Decrease [132] | NC |
| Administer Midazolam - 5 mg | Drug Onset in 1-3 min and lasts 30-60 minutes | 30 | 70 | Mild Increase [126] p200; 5-10% Decrease [253] | Moderate Decrease [126] p200; 15-25% Decrease [253] | Moderate Decrease [126] p200; 15-25% Decrease [253] | Moderate Decrease [126] p200; Marked Decrease To 0 [253] | As long as some respiration rate, little change. If RR=0, then begins to drop along O2 curve. |
| Administer Morphine- 42 mg | Drug Onset in less than 2 minutes | 30 | 100 | Mild Decrease [126] p200; 5-10% Decrease [253] | Decrease - dose dependent [126] p200; 5-10% Decrease [253] | Decrease - dose dependent [126] p200; 5-10% Decrease [253] | Marked Decrease [126] p200; 15-25% Decrease [253] | As long as some respiration rate, little change. If RR=0, then begins to drop along O2 curve. |
| Administer Fentanyl - 17 mg | Drug Onset < two minutes | 30 | 100 | Stable [126] p192-7, [265] p277; 5-10% Decrease [253] | Stable [126] p192-7, [265] p277; 5-10% Decrease [253] | Stable [126] p192-7, [265] p277; 5-10% Decrease [253] | Decrease [126] p192-7, [265] p277; 15-25% Decrease [253] | NC |
| Administer Naloxone - 30ug | Drug Onset in one to two minutes | 330 | 350 | NC or Increase [126] p192-7, [265] p285; 5-10% Increase [253] | NC or Increase [126] p192-7, [265] p285; 5-10% Increase [253] | NC or Increase [126] p192-7, [265] p285; 5-10% Increase [253] | Decrease [126] p192-7, [265] p285; Return to resting physiology [253] | NC or Increase |
| Administer Pralidoxime - 135 mg | Drug Onset in less than 1 minute | 30 | 100 | Tachycardia [310] | Increased [310] | Increased [310] | Increased [310] | NC [311] |
| Administer Prednisone - 135 mg | Drug Onset in less than 1 minute | 30 | 100 | NC [311] | NC [311] | NC [311] | NC [311] | NC [311] |
| Administer Propofol - 135 mg | Drug Onset in less than 1 minute | 30 | 100 | NC [126] p200; NC [253] | Marked Decrease [126] p200; 25-40% Decrease [253] | Marked Decrease [126] p200; 25-40% Decrease [253] | Marked Decrease [126] p200; 100% Decrease [253] | Begins to drop according to O2 curve |
| Administer Rocuronium - 52 mg | Drug Onset in 60-90 seconds | 30 | 100 | NC [265] p299; NC [253] | NC [265] p299; NC [253] | NC [265] p299; NC [253] | Goes to Zero [126] p224; Goes to Zero [253] | Begins to drop according to O2 curve |
| Administer Succinylcholine - 96 mg | Drug Onset < 1 minute | 30 | 100 | Mild Increase [126] pp210-5; 5-10% Decrease [253] | Mild Increase [126] pp210-5; 5-10% Decrease [253] | Mild Increase [126] pp210-5; 5-10% Decrease [253] | Goes to Zero [126] p224; Goes to Zero [253] | Begins to drop according to O2 curve |
| Event | Notes | Action Occurance Time (s) | Sample Scenario Time (s) | Heart Rate (beats/min) | Systolic Pressure (mmHg) | Diastolic Pressure (mmHg) | Respiration Rate (breaths/min) | Tidal Volume (mL) | Oxygen Saturation |
|---|---|---|---|---|---|---|---|---|---|
| Administer Albuterol - 10ug | Drug Onset < 1 minute | 30 | 200 | 25-50% Increase [144] | Slight Increase [144] | Slight Increase [144] | NC [144] | NC [144] | NC |
| Event | Notes | Action Occurance Time (s) | Sample Scenario Time (min) | Urine Production Rate (mL/min) | Chloride Excretion (mmol/min) | Sodium Excretion (mol/min) | Vascular Volume (mL) |
|---|---|---|---|---|---|---|---|
| Administer Furosemide - 40 mg | Drug Onset < 1 minute | 30 | 450 | 300-400% Increase [201] | 300-400% Increase [201] | 300-400% Increase [201] | Decrease (around 500-1000 mL) [152] |
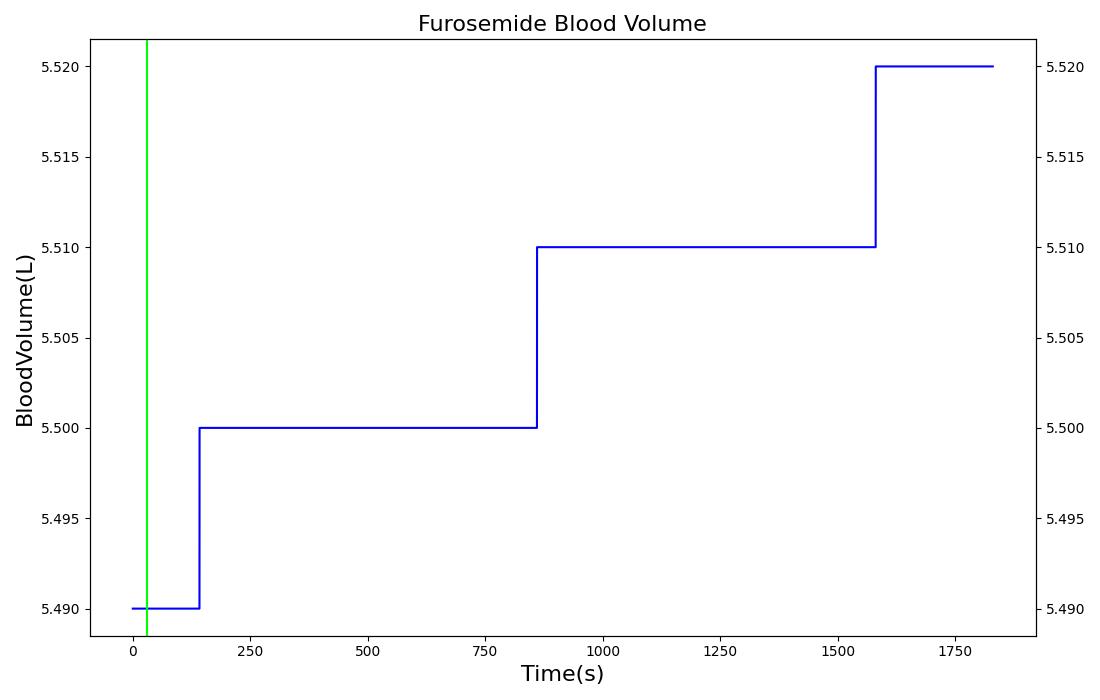
Diuretic-Furosemide
PD effects for the diuretic Furosemide are handled differently than other drugs in the engine. Localized effects are implemented via a tubular permeability modifier. This effectively simulates Furosemide's effect on the tubular luminal Na-K-Cl co-transporter, inhibiting reabsorption of fluid and ions into the vasculature, as shown in Figure 15. The permeability modifier is a function of the PK effects of the drug concentration in the blood plasma. These concentrations are then mapped to a Hill-type sigmoid to determine appropriate tubular reabsorption inhibition via reducing the permeability of the tubular lumen, see Renal Methodology. This effectively increases the urine production rate and ion excretion, and reduces the patient's total blood volume. To see the effects of the drug on the cardiovascular system during ventricular systolic dysfunction, see Cardiovascular System.
 |  |
 |  |
 | |
Norepinephrine occurs naturally in the body and its basal metabolic value is validated as part of the Blood Chemistry Methodology. The infusion of norepinephrine was validated for the plasma concentration and the effects on heart rate and systolic and diastolic pressure. The values were examined for five different infusion rates. They are shown in Table 9.
| Drug | Infusion Rate (ug/(kg min)) | Experimental Plasma Concentration (ug/L) | Computed Plasma Concentration (ug/L) | Experimental Heart Rate (beats/min) | Computed Heart Rate (beats/min) | Experimental Systolic Blood Pressure (mmHg) | Computed Systolic Blood Pressure (mmHg) | Experimental Diastolic Blood Pressure (mmHg) | Computed Diastolic Blood Pressure (mmHg) |
|---|---|---|---|---|---|---|---|---|---|
| Norepinephrine | 0.01 | [0.363, 0.605] [100] | 0.761 | [46, 66] NC to neglible [100] | 73 | [125, 145] Minimal Increase [100] | 118 | [65, 75] NC [100] | 72 |
| Norepinephrine | 0.06 | [2.265, 3.391] [100] | 2.32 | [41, 57]Minimal Decrease [100] | 70 | [136, 160] Minimal Increase [100] | 118 | [70, 84] Minimal Increase [100] | 72 |
| Norepinephrine | 0.1 | [3.608, 4.408] [100] | 3.71 | [40, 56] Minimal Decrease [100] | 68 | [146, 168] Minimal Increase [100] | 125 | [74, 86] Minimal Increase [100] | 84 |
| Norepinephrine | 0.14 | [4.619, 6.765] [100] | 5.05 | [39, 55] Minimal Decrease[100] | 69 | [152, 182] Minimal Increase [100] | 135 | [77, 93] Minimal Increase [100] | 98 |
| Norepinephrine | 0.2 | [6.404, 8.546] [100] | 7.1 | [39, 55] NC [100] | 69 | [174, 192] Minimal Increase [100] | 136 | [83, 99] Minimal Increase [100] | 100 |
The infusion of phenylephrine was validated for the plasma concentration and the effects on heart rate and systolic and diastolic pressure. The values were examined for four different infusion rates. They are shown in Table 10.
| Drug | Infusion Rate (ug/(kg min)) | Experimental Plasma Concentration (ug/L) | Computed Plasma Concentration (ug/L) | Experimental Heart Rate Change (beats/min) | Computed Heart Rate Change (beats/min) | Experimental Systolic Blood Pressure (mmHg) | Computed Systolic Blood Pressure (mmHg) | Experimental Diastolic Blood Pressure (mmHg) | Computed Diastolic Blood Pressure (mmHg) |
|---|---|---|---|---|---|---|---|---|---|
| Phenylephrine | 0.5 | [2.5, 5.2] [221] | 6 | [1, -5] [221] | -2 | [0, 6] [221] | 6 | [2, 9] [221] | 9 |
| Phenylephrine | 1 | [5.02, 13.0] [221] | 12.4 | [-3, -8] [221] | -2 | [6, 20] [221] | 9 | [9, 15] [221] | 13 |
| Phenylephrine | 2 | [12.4, 26.3] [221] | 24.9 | [-8, -12] [221] | -1 | [16, 53] [221] | 13 | [15, 21] [221] | 19 |
| Phenylephrine | 4 | [35.6, 77.8] [221] | 48.4 | [-10, -13] [221] | 2 | [30, 86] [221] | 17 | [25, 32] [221] | 25 |
Conclusions
In general, the drugs in the data library have strong agreement with both the published data and subject matter expertise. The biggest limitation lies in the transient response to drugs. Currently, the system tolerances provide a response within 30-60 seconds for all drugs, regardless of the physiologic onset time. The drug response also wears off quickly for all drugs. We plan to improve our determination of the EC50 parameter. The EC50 values are too high for each drug leading to an early spike corresponding to the spike in plasma concentration and rapid diffusion into the tissues. We will incorporate a minimum diffusion amount, then determine the EC50 parameter to improve the onset and duration of effects. We will also add a suppression of the chemoreceptor responses due to sedation in the future. We plan to add more drugs to the library that are relevant to sedation and pain management. We are also investigating the implementation of an intoxication model, as our current model only represents the "normal" drug response range.
These models and the combination of these models represent a positive step forward for drug simulation. The parameters are intuitive and accurate for each drug and additional drugs can be added for an expanded drug library.
Future Work
Coming Soon
- Intoxication
- Expanded drug library
- Improved drug onset and duration times
Recommended Improvements
- Combinatory effects of kinetics
- Long-term drug effects, including physiological changes
- Additional drugs
- Improved kinetic model for aerosols and vapors
- Higher fidelity models of other routes, including epithelial cell (integumentary, gastrointestinal) absorption
- Drug sensitivity variation
Appendices
Acronyms
MAP - Mean Arterial Pressure
PK - Pharmacokinetic
PD - Pharmacodynamic
SDK - Software Development Kit
SMEs - Subject Matter Experts